EMID2 is a novel biotherapeutic for aggressive cancers identified by in vivo screening
- PMID: 38195652
- PMCID: PMC10777502
- DOI: 10.1186/s13046-023-02942-4
EMID2 is a novel biotherapeutic for aggressive cancers identified by in vivo screening
Abstract
Background: New drugs to tackle the next pathway or mutation fueling cancer are constantly proposed, but 97% of them are doomed to fail in clinical trials, largely because they are identified by cellular or in silico screens that cannot predict their in vivo effect.
Methods: We screened an Adeno-Associated Vector secretome library (> 1000 clones) directly in vivo in a mouse model of cancer and validated the therapeutic effect of the first hit, EMID2, in both orthotopic and genetic models of lung and pancreatic cancer.
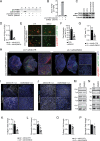
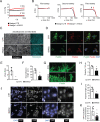
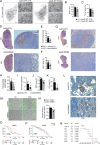
Results: EMID2 overexpression inhibited both tumor growth and metastatic dissemination, consistent with prolonged survival of patients with high levels of EMID2 expression in the most aggressive human cancers. Mechanistically, EMID2 inhibited TGFβ maturation and activation of cancer-associated fibroblasts, resulting in more elastic ECM and reduced levels of YAP in the nuclei of cancer cells.
Conclusion: This is the first in vivo screening, precisely designed to identify proteins able to interfere with cancer cell invasiveness. EMID2 was selected as the most potent protein, in line with the emerging relevance of the tumor extracellular matrix in controlling cancer cell invasiveness and dissemination, which kills most of cancer patients.
Keywords: AAV vectors; Biotherapeutics; Cancer; Cell invasiveness; Gene therapy; In vivo screening.
© 2024. The Author(s).
Conflict of interest statement
MG is a founder, member of the board, equity holder, and consultant for Forcefield Therapeutics, London, FB is a consultant of the same company. The AAV secretome library and the FunSel technology are owned by Purespring Therapeutics, for which MG is a cofounder, observer to the board, equity holder, and consultant.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

