Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial
- PMID: 38195752
- PMCID: PMC10878978
- DOI: 10.1038/s41591-023-02760-3
Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial
Abstract
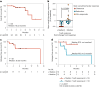
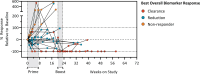
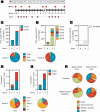
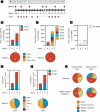
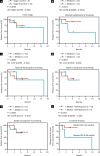
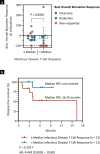
Pancreatic and colorectal cancers are often KRAS mutated and are incurable when tumor DNA or protein persists or recurs after curative intent therapy. Cancer vaccine ELI-002 2P enhances lymph node delivery and immune response using amphiphile (Amph) modification of G12D and G12R mutant KRAS (mKRAS) peptides (Amph-Peptides-2P) together with CpG oligonucleotide adjuvant (Amph-CpG-7909). We treated 25 patients (20 pancreatic and five colorectal) who were positive for minimal residual mKRAS disease (ctDNA and/or serum tumor antigen) after locoregional treatment in a phase 1 study of fixed-dose Amph-Peptides-2P and ascending-dose Amph-CpG-7909; study enrollment is complete with patient follow-up ongoing. Primary endpoints included safety and recommended phase 2 dose (RP2D). The secondary endpoint was tumor biomarker response (longitudinal ctDNA or tumor antigen), with exploratory endpoints including immunogenicity and relapse-free survival (RFS). No dose-limiting toxicities were observed, and the RP2D was 10.0 mg of Amph-CpG-7909. Direct ex vivo mKRAS-specific T cell responses were observed in 21 of 25 patients (84%; 59% both CD4+ and CD8+); tumor biomarker responses were observed in 21 of 25 patients (84%); biomarker clearance was observed in six of 25 patients (24%; three pancreatic and three colorectal); and the median RFS was 16.33 months. Efficacy correlated with T cell responses above or below the median fold increase over baseline (12.75-fold): median tumor biomarker reduction was -76.0% versus -10.2% (P < 0.0014), and the median RFS was not reached versus 4.01 months (hazard ratio = 0.14; P = 0.0167). ELI-002 2P was safe and induced considerable T cell responses in patients with immunotherapy-recalcitrant KRAS-mutated tumors. ClinicalTrials.gov identifier: NCT04853017 .
© 2024. The Author(s).
Conflict of interest statement
S.P. reports clinical research funding to institution from Arqule, Bristol Myers Squibb, Eli Lilly, Elicio Therapeutics, Holy Stone Healthcare, Ipsen, Mirati Therapeutics, Novartis, Rgenix, Sanofi-Aventis, Xencor, Astellas, Framewave, 4D Pharma, Boehringer Ingelheim, NGM Biopharmaceuticals, Janssen, Arcus Biosciences, BioNTech, Zymeworks and Pfizer. S.P. also reports consultant/advisory fees from Zymeworks, Ipsen, Novartis, Janssen and Boehringer Ingelheim. Z.A.W. has received consultant/advisory fees from Lilly Oncology, AstraZeneca, Merck, Daiichi Sankyo, Macrogenics, Amgen, Bristol Myers Squibb, Astellas, Ipsen, Arcus, Novartis, Roche, Seagen and Pfizer and has received research funding (institutional) from Elicio Therapeutics, Arcus, Plexxikon, Novartis and Merck. C.D.W. reports research funding from Elicio Therapeutics, Novartis, Actuate Therapeutics, Merck, AstraZeneca and Mirati and advisory board relationships with Ipsen, Actuate Therapeutics and Genentech. M.F. has received research funding (institutional) from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, Genmab, Elicio Therapeutics, Mirati, Amgen, Replimmune, Checkmate Pharmaceuticals, Gilead, GlaxoSmithKline, Immunocore, Seagen, Tesaro and Abbvie and has been on advisory boards for Abbvie, Beigene, Jazz Pharmaceuticals, Mirati and AstraZeneca. P.M.K. reports a consultancy/advisory board relationship with Elicio (scientific advisory board member/stock ownership); consultancy/advisory board relationships with Guardant Health, Illumina, Natera, Foundation Medicine, Daichi Sankyo, Tempus, Bayer, MSD Oncology/Merck, Delcath Systems, QED Therapeutics, Taiho Oncology (self/institution), Exact Sciences, Eisai, BostonGene, Neogenomics, Saga Diagnostics, Servier, Seagen, Eli Lilly and Ipsen (to institution); and research funding/trial support from Merck (to institution), Novartis (to institution), Agenus Bio (to institution), Boston Scientific (to institution), Tersera (to institution) and Advanced Accelerator Applications as well as a travel grant for IIT from AstraZeneca. C.E.D. reports research funding from Elicio Therapeutics. A.D.L. reports institutional contracts with Elicio Therapeutics, Bristol Myers Squibb, Exelixis, Arrys Therapeutics, Hutchison Medipharma, Corcept Therapeutics, Conjupro Biotherapeutics and AbbVie for trials where she is the local principal investigator. She serves as Pancreatic Cancer/Neuroendocrine Co-Chair on the Elsevier ClinicalPath Oncology Committee. V.C. has held a consulting or advisory role at Ispen, Gristone Oncology, Westwood Bioscience and Apeiron Biologics; he has been on the speakers’ bureau for Ipsen and Celgene; and he has received research funding from Elicio Therapeutics, Roche and Merck. O.B. reports no relevant disclosures. H.V., L.M.S., A.M.T., J.R.P., E.W., L.K.M., P.C.D., T.K. and C.M.H. are current or former employees of Elicio Therapeutics and, as such, receive salary and benefits, including ownership of stock and stock options, from the company. L.K.M., P.C.D. and C.M.H. have amphiphile vaccine patents pending to Elicio Therapeutics. E.M.O. reports relationships with Rafael Therapeutics (DSMB); Seagen, Boehringer Ingelheim, BioNTech, Ipsen, Merck, Silenseed, Novartis, AstraZeneca, BioSapien, Thetis and Autem, Tempus (consulting/advisory relationship); Agios, Genentech/Roche, Eisai, Zymeworks (spouse: consulting/advisory relationship); and Genentech/Roche, Celgene/Bristol Myers Squibb, BioNTech, AstraZeneca, Arcus, Elicio Therapeutics, Parker Institute, Pertzye and NCI/NIH (research funding).
Figures





Comment in
-
A new era with advanced immunotherapy.Transl Gastroenterol Hepatol. 2024 Sep 24;9:60. doi: 10.21037/tgh-24-37. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 39503024 Free PMC article. No abstract available.
-
A new dawn in cancer immunotherapy: the promise of mutant KRAS-specific vaccines.Transl Gastroenterol Hepatol. 2025 Apr 10;10:20. doi: 10.21037/tgh-24-121. eCollection 2025. Transl Gastroenterol Hepatol. 2025. PMID: 40337764 Free PMC article. No abstract available.
References
-
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin.71, 7–33 (2021). - PubMed
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin.70, 7–30 (2020). - PubMed
-
- Modest, D. P., Pant, S. & Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer109, 70–83 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

