Potential and Uncertainties of RejectClass in Acute Kidney Graft Dysfunction: An Independent Validation Study
- PMID: 38196094
- PMCID: PMC11042522
- DOI: 10.1097/TP.0000000000004906
Potential and Uncertainties of RejectClass in Acute Kidney Graft Dysfunction: An Independent Validation Study
Abstract
Background: Kidney graft rejections are classified based on the Banff classification. The RejectClass algorithm, initially derived from a cohort comprising mostly protocol biopsies, identifies data-driven phenotypes of acute rejection and chronic pathology using Banff lesion scores. It also provides composite scores for inflammation activity and chronicity. This study independently evaluates the performance of RejectClass in a cohort consisting entirely of indication biopsies.
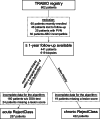
Methods: We retrospectively applied RejectClass to 441 patients from the German TRABIO (TRAnsplant BIOpsies) cohort who had received indication biopsies. The primary endpoint was death-censored graft failure during 2 y of follow-up.
Results: The application of RejectClass to our cohort demonstrated moderately comparable phenotypic features with the derivation cohort, and most clusters indicated an elevated risk of graft loss. However, the reproduction of all phenotypes and the associated risks of graft failure, as depicted in the original studies, was not fully accomplished. In contrast, adjusted Cox proportional hazards analyses substantiated that both the inflammation score and the chronicity score are independently associated with graft loss, exhibiting hazard ratios of 1.7 (95% confidence interval, 1.2-2.3; P = 0.002) and 2.2 (95% confidence interval, 1.8-2.6; P < 0.001), respectively, per 0.25-point increment (scale: 0.0-1.0).
Conclusions: The composite inflammation and chronicity scores may already have direct utility in quantitatively assessing the disease stage. Further refinement and validation of RejectClass clusters are necessary to achieve more reliable and accurate phenotyping of rejection.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
F.A.v.S.-H. and B.K. are supported by the Medical Faculty of the Christian-Albrechts-University Kiel. J.H.B. and J.S. were supported by the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung [BMBF], KMU-innovativ: grant 13GW0399B). H.U.Z. was supported by the BMBF within the framework of the e:Med research and funding concept (grant 01ZX1912A). The other authors declare no conflicts of interest.
Figures




Comment in
-
Phenotypes and Prognostic Subgroups Derived by the RejectClass Clustering Algorithm Are Not Fully Reproducible in an Independent Multicenter Study.Transplantation. 2024 May 1;108(5):1060-1061. doi: 10.1097/TP.0000000000004907. Epub 2024 Jan 10. Transplantation. 2024. PMID: 38196110 No abstract available.
References
-
- Tonelli M, Wiebe N, Knoll G, et al. . Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. - PubMed
-
- Sellarés J, de Freitas DG, Mengel M, et al. . Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

