Theoretical hypothesis in a direct electron transfer between non-interacting Fe-S proteins within an artificial fusion
- PMID: 38196139
- PMCID: PMC10795574
- DOI: 10.1093/femsle/fnad137
Theoretical hypothesis in a direct electron transfer between non-interacting Fe-S proteins within an artificial fusion
Abstract
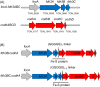
Reduction of CO2 to formate utilizing formate dehydrogenases (FDHs) has been attempted biologically and electrochemically. However, the conversion efficiency is very low due to the low energy potential of electron donors and/or electron competition with other electron acceptors. To overcome such a low conversion efficiency, I focused on a direct electron transfer between two unrelated redox enzymes for the efficient reduction of CO2 and utilized the quantum mechanical magnetic properties of the [Fe-S] ([iron-sulfur]) cluster to develop a novel electron path. Using this electron path, we connected non-interacting carbon monoxide dehydrogenase and FDH, constructing a synthetic carbon monoxide:formate oxidoreductase as a single functional enzyme complex in the previous study. Here, a theoretical hypothesis that can explain the direct electron transfer phenomenon based on the magnetic properties of the [Fe-S] cluster is proposed.
Keywords: Fe-S protein; biorefinery; carbon monoxide:formate oxidoreductase; direct electron transfer; protein fusion; synthetic enzyme.
© The Author(s) 2024. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Bioconversion of CO to formate by artificially designed carbon monoxide:formate oxidoreductase in hyperthermophilic archaea.Commun Biol. 2022 Jun 3;5(1):539. doi: 10.1038/s42003-022-03513-7. Commun Biol. 2022. PMID: 35660788 Free PMC article.
-
Synthesis of Formate from CO2 Gas Catalyzed by an O2-Tolerant NAD-Dependent Formate Dehydrogenase and Glucose Dehydrogenase.Biochemistry. 2019 Apr 9;58(14):1861-1868. doi: 10.1021/acs.biochem.8b01301. Epub 2019 Mar 18. Biochemistry. 2019. PMID: 30839197
-
Understanding How the Rate of C-H Bond Cleavage Affects Formate Oxidation Catalysis by a Mo-Dependent Formate Dehydrogenase.J Am Chem Soc. 2020 Jul 15;142(28):12226-12236. doi: 10.1021/jacs.0c03574. Epub 2020 Jul 6. J Am Chem Soc. 2020. PMID: 32551568 Free PMC article.
-
Molybdenum and tungsten-dependent formate dehydrogenases.J Biol Inorg Chem. 2015 Mar;20(2):287-309. doi: 10.1007/s00775-014-1218-2. Epub 2014 Dec 5. J Biol Inorg Chem. 2015. PMID: 25476858 Review.
-
Classification and enzyme kinetics of formate dehydrogenases for biomanufacturing via CO2 utilization.Biotechnol Adv. 2019 Nov 15;37(7):107408. doi: 10.1016/j.biotechadv.2019.06.007. Epub 2019 Jun 12. Biotechnol Adv. 2019. PMID: 31200015 Review.
Cited by
-
Stabilization of the catalytically active structure of a molybdenum-dependent formate dehydrogenase depends on a highly conserved lysine residue.FEBS J. 2025 Jun;292(12):3165-3179. doi: 10.1111/febs.70048. Epub 2025 Mar 3. FEBS J. 2025. PMID: 40028997 Free PMC article.
References
-
- Beinert H, Holm RH, Münck E. Iron-sulfur clusters: nature’s modular, multipurpose structures. Science. 1997;277:653–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

