Adhesive properties of Aphrophoridae spittlebug foam
- PMID: 38196374
- PMCID: PMC10777165
- DOI: 10.1098/rsif.2023.0521
Adhesive properties of Aphrophoridae spittlebug foam
Abstract
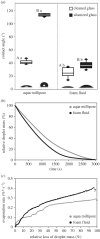
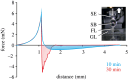
Aphrophora alni spittlebug nymphs produce a wet foam from anal excrement fluid, covering and protecting themselves against numerous impacts. Foam fluid contact angles on normal (26°) and silanized glass (37°) suggest that the foam wets various substrates, including plant and arthropod surfaces. The pull-off force depends on the hydration state and is higher the more dry the fluid. Because the foam desiccates as fast as water, predators once captured struggle to free from drying foam, becoming stickier. The present study confirms that adhesion is one of the numerous foam characteristics resulting in multifunctional effects, which promote spittlebugs' survival and render the foam a smart, biocompatible material of biological, biomimetic and biomedical interest. The sustainable 'reuse' of large amounts of excrement for foam production and protection of the thin nymph integument suggests energetic and evolutionary advantages. Probably, that is why foam nests have evolved in different groups of organisms, such as spittlebugs, frogs and fish.
Keywords: cryo-SEM; defence; foam nest; insect cuticle; pull-off force; surfactant.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Cantat I, Cohen-Addad S, Elias F, Graner F, Höhler R, Pitois O, Rouyer F, Saint-Jalmes A. 2013. Foams. Structure and dynamics. Oxford, UK: Oxford University Press.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
