This is a preprint.
Structure of the dopamine D3 receptor bound to a bitopic agonist reveals a new specificity site in an expanded allosteric pocket
- PMID: 38196573
- PMCID: PMC10775388
- DOI: 10.21203/rs.3.rs-3433207/v1
Structure of the dopamine D3 receptor bound to a bitopic agonist reveals a new specificity site in an expanded allosteric pocket
Update in
-
A bitopic agonist bound to the dopamine 3 receptor reveals a selectivity site.Nat Commun. 2024 Sep 5;15(1):7759. doi: 10.1038/s41467-024-51993-4. Nat Commun. 2024. PMID: 39237617 Free PMC article.
Abstract
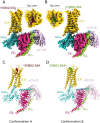
Although aminergic GPCRs are the target for ~25% of approved drugs, developing subtype selective drugs is a major challenge due to the high sequence conservation at their orthosteric binding site. Bitopic ligands are covalently joined orthosteric and allosteric pharmacophores with the potential to boost receptor selectivity, driven by the binding of the secondary pharmacophore to non-conserved regions of the receptor. Although bitopic ligands have great potential to improve current medications by reducing off-target side effects, the lack of structural information on their binding mode impedes rational design. Here we determine the cryo-EM structure of the hD3R coupled to a GO heterotrimer and bound to the D3R selective bitopic agonist FOB02-04A. Structural, functional and computational analyses provide new insights into its binding mode and point to a new TM2-ECL1-TM1 region, which requires the N-terminal ordering of TM1, as a major determinant of subtype selectivity in aminergic GPCRs. This region is underexploited in drug development, expands the established secondary binding pocket in aminergic GPCRs and could potentially be used to design novel and subtype selective drugs.
Keywords: G protein-coupled receptors; GPCR; bitopic drugs; cryo-EM; dopamine D3 receptor; drug selectivity.
Figures




References
-
- Lane J. R., Sexton P. M. & Christopoulos A. Bridging the gap: Bitopic ligands of G-protein-coupled receptors. Trends Pharmacol Sci 34, 59–66 (2013). - PubMed
-
- Valant C., Robert Lane J., Sexton P. M. & Christopoulos A. The best of both worlds? bitopic orthosteric/allosteric ligands of g proteincoupled receptors. Annu Rev Pharmacol Toxicol 52, 153–178 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

