This is a preprint.
Sex differences in mitochondrial gene expression during viral myocarditis
- PMID: 38196574
- PMCID: PMC10775395
- DOI: 10.21203/rs.3.rs-3716881/v1
Sex differences in mitochondrial gene expression during viral myocarditis
Update in
-
Sex differences in mitochondrial gene expression during viral myocarditis.Biol Sex Differ. 2024 Dec 18;15(1):104. doi: 10.1186/s13293-024-00678-0. Biol Sex Differ. 2024. PMID: 39696682 Free PMC article.
Abstract
Background: Myocarditis is an inflammation of the heart muscle most often caused by an immune response to viral infections. Sex differences in the immune response during myocarditis have been well described but upstream mechanisms in the heart that might influence sex differences in disease are not completely understood.
Methods: Male and female BALB/c wild type mice received an intraperitoneal injection of heart-passaged coxsackievirus B3 (CVB3) or vehicle control. Bulk-tissue RNA-sequencing was conducted to better understand sex differences in CVB3 myocarditis. We performed enrichment analysis to understand sex differences in the transcriptional landscape of myocarditis and identify candidate transcription factors that might drive sex differences in myocarditis.
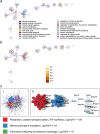
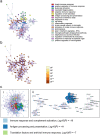
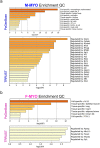
Results: The hearts of male and female mice with myocarditis were significantly enriched for pathways related to an innate and adaptive immune response compared to uninfected controls. When comparing females to males with myocarditis, males were enriched for inflammatory pathways and gene changes that suggested worse mitochondrial transcriptional support (e.g., mitochondrial electron transport genes). In contrast, females were enriched for pathways related to mitochondrial respiration and bioenergetics, which were confirmed by higher transcript levels of master regulators of mitochondrial function including peroxisome proliferator-activated receptor gamma coactivator 1 (PGC1α), nuclear respiratory factor 1 (NRF1) and estrogen-related receptor alpha (ERRα). TRANSFAC analysis identified ERRa as a transcription factor that may mediate sex differences in mitochondrial function during myocarditis.
Conclusions: Master regulators of mitochondrial function were elevated in females with myocarditis compared to males and may promote sex differences in mitochondrial respiratory transcript expression during viral myocarditis resulting in less severe myocarditis in females following viral infection.
Keywords: autoimmune disease; coxsackievirus B3; estrogen-related receptor alpha estrogen/ERRa; inflammation; innate immunity; interleukin-1 beta; mitochondria; nuclear respiratory factor 1/NRF1; peroxisome proliferator-activated receptor gamma coactivator 1/PGC1a.
Conflict of interest statement
Competing Interests The authors declare that they have no competing interests.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

