This is a preprint.
Lentiviral mediated delivery of CRISPR/Cas9 reduces intraocular pressure in a mouse model of myocilin glaucoma
- PMID: 38196579
- PMCID: PMC10775399
- DOI: 10.21203/rs.3.rs-3740880/v1
Lentiviral mediated delivery of CRISPR/Cas9 reduces intraocular pressure in a mouse model of myocilin glaucoma
Update in
-
Lentiviral mediated delivery of CRISPR/Cas9 reduces intraocular pressure in a mouse model of myocilin glaucoma.Sci Rep. 2024 Mar 23;14(1):6958. doi: 10.1038/s41598-024-57286-6. Sci Rep. 2024. PMID: 38521856 Free PMC article.
Abstract
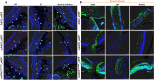
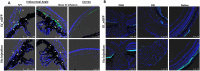
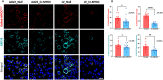
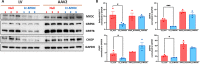
Mutations in myocilin (MYOC) are the leading known genetic cause of primary open-angle glaucoma, responsible for about 4% of all cases. Mutations in MYOC cause a gain-of-function phenotype in which mutant myocilin accumulates in the endoplasmic reticulum (ER) leading to ER stress and trabecular meshwork (TM) cell death. Therefore, knocking out myocilin at the genome level is an ideal strategy to permanently cure the disease. We have previously utilized CRISPR/Cas9 genome editing successfully to target MYOC using adenovirus 5 (Ad5). However, Ad5 is not a suitable vector for clinical use. Here, we sought to determine the efficacy of adeno-associated viruses (AAVs) and lentiviruses (LVs) to target the TM. First, we examined the TM tropism of single-stranded (ss) and self-complimentary (sc) AAV serotypes as well as LV expressing GFP via intravitreal (IVT) and intracameral (IC) injections. We observed that LV_GFP expression was more specific to the TM injected via the IVT route. IC injections of Trp-mutant scAAV2 showed a prominent expression of GFP in the TM. However, robust GFP expression was also observed in the ciliary body and retina. We next constructed lentiviral particles expressing Cas9 and guide RNA (gRNA) targeting MYOC (crMYOC) and transduction of TM cells stably expressing mutant myocilin with LV_crMYOC significantly reduced myocilin accumulation and its associated chronic ER stress. A single IVT injection of LV_crMYOC in Tg-MYOCY437H mice decreased myocilin accumulation in TM and reduced elevated IOP significantly. Together, our data indicates, LV_crMYOC targets MYOC gene editing in TM and rescues a mouse model of myocilin-associated glaucoma.
Keywords: ER stress; Gene therapy; Genome editing for glaucoma; Intraocular pressure; Lentiviral particles; Myocilin-associated Glaucoma; Trabecular meshwork; Viral vectors.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

