This is a preprint.
Skin-inspired, sensory robots for electronic implants
- PMID: 38196588
- PMCID: PMC10775366
- DOI: 10.21203/rs.3.rs-3665801/v1
Skin-inspired, sensory robots for electronic implants
Update in
-
Skin-inspired, sensory robots for electronic implants.Nat Commun. 2024 Jun 5;15(1):4777. doi: 10.1038/s41467-024-48903-z. Nat Commun. 2024. PMID: 38839748 Free PMC article.
Abstract
Living organisms with motor and sensor units integrated seamlessly demonstrate effective adaptation to dynamically changing environments. Drawing inspiration from cohesive integration of skeletal muscles and sensory skins in these organisms, we present a design strategy of soft robots, primarily consisting of an electronic skin (e-skin) and an artificial muscle, that naturally couples multifunctional sensing and on-demand actuation in a biocompatible platform. We introduce an in situ solution-based method to create an e-skin layer with diverse sensing materials (e.g., silver nanowires, reduced graphene oxide, MXene, and conductive polymers) incorporated within a polymer matrix (e.g., polyimide), imitating complex skin receptors to perceive various stimuli. Biomimicry designs (e.g., starfish and chiral seedpods) of the robots enable various motions (e.g., bending, expanding, and twisting) on demand and realize good fixation and stress-free contact with tissues. Furthermore, integration of a battery-free wireless module into these robots enables operation and communication without tethering, thus enhancing the safety and biocompatibility as minimally invasive implants. Demonstrations range from a robotic cuff encircling a blood vessel for detecting blood pressure, to a robotic gripper holding onto a bladder for tracking bladder volume, an ingestible robot residing inside stomach for pH sensing and on-site drug delivery, and a robotic patch wrapping onto a beating heart for quantifying cardiac contractility, temperature and applying cardiac pacing, highlighting the application versatilities and potentials of the nature-inspired soft robots. Our designs establish a universal strategy with a broad range of sensing and responsive materials, to form integrated soft robots for medical technology and beyond.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures
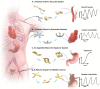





References
-
- Xiao X., Xiao X., Lan Y., Chen J., Learning from nature for healthcare, energy, and environment, Innov. 2 (2021) 100135. https://doi.org/10.1016/j.xinn.2021.100135. - DOI - PMC - PubMed
-
- Wolf C., Linden D.E.J., Biological pathways to adaptability – interactions between genome, epigenome, nervous system and environment for adaptive behavior, Genes, Brain Behav. 11 (2012) 3–28. https://doi.org/10.1111/j.1601-183X.2011,00752.x. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

