This is a preprint.
Lysine-36 of Drosophila histone H3.3 supports adult longevity
- PMID: 38196611
- PMCID: PMC10775331
- DOI: 10.1101/2023.09.28.559962
Lysine-36 of Drosophila histone H3.3 supports adult longevity
Update in
-
Lysine-36 of Drosophila histone H3.3 supports adult longevity.G3 (Bethesda). 2024 Apr 3;14(4):jkae030. doi: 10.1093/g3journal/jkae030. G3 (Bethesda). 2024. PMID: 38366796 Free PMC article.
Abstract
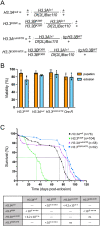
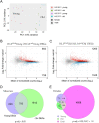
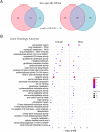
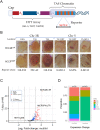
Aging is a multifactorial process that disturbs homeostasis, increases disease susceptibility, and ultimately results in death. Although the definitive set of molecular mechanisms responsible for aging remain to be discovered, epigenetic change over time is proving to be a promising piece of the puzzle. Several posttranslational histone modifications (PTMs) have been linked to the maintenance of longevity. Here, we focus on lysine-36 of the replication-independent histone protein, H3.3 (H3.3K36). To interrogate the role of this residue in Drosophila developmental gene regulation, we generated a lysine to arginine mutant that blocks the activity of its cognate modifying enzymes. We found that an H3.3BK36R mutation causes a significant reduction in adult lifespan, accompanied by dysregulation of the genomic and transcriptomic architecture. Transgenic co-expression of wild-type H3.3B completely rescues the longevity defect. Because H3.3 is known to accumulate in non-dividing tissues, we carried out transcriptome profiling of young vs aged adult fly heads. The data show that loss of H3.3K36 results in age-dependent misexpression of NF-κB and other innate immune target genes, as well as defects in silencing of heterochromatin. We propose H3.3K36 maintains the postmitotic epigenomic landscape, supporting longevity by regulating both pericentric and telomeric retrotransposons and by suppressing aberrant immune signaling.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
