High Population Frequency of GNRHR p.Q106R in Malta: An Evaluation of Fertility and Hormone Profiles in Heterozygotes
- PMID: 38196663
- PMCID: PMC10775685
- DOI: 10.1210/jendso/bvad172
High Population Frequency of GNRHR p.Q106R in Malta: An Evaluation of Fertility and Hormone Profiles in Heterozygotes
Abstract
Context: The gonadotropin-releasing hormone receptor variant GNRHR p.Q106R (rs104893836) in homozygosity, compound heterozygosity, or single heterozygosity is often reported as the causative variant in idiopathic hypogonadotropic hypogonadism (IHH) patients with GnRH deficiency. Genotyping of a Maltese newborn cord-blood collection yielded a minor allele frequency (MAF) 10 times higher (MAF = 0.029; n = 493) than that of the global population (MAF = 0.003).
Objective: To determine whether GNRHR p.Q106R in heterozygosity influences profiles of endogenous hormones belonging to the hypothalamic-pituitary axis and the onset of puberty and fertility in adult men (n = 739) and women (n = 239).
Design setting and participants: Analysis of questionnaire data relating to puberty and fertility, genotyping of the GNRHR p.Q106R variant, and hormone profiling of a highly phenotyped Maltese adult cohort from the Maltese Acute Myocardial Infarction Study.
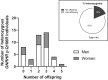
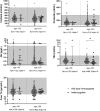
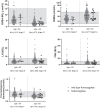
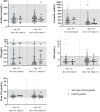
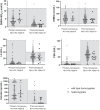
Main outcome and results: Out of 978 adults, 43 GNRHR p.Q106R heterozygotes (26 men and 17 women) were identified. Hormone levels and fertility for all heterozygotes are within normal parameters except for TSH, which was lower in men 50 years or older.
Conclusion: Hormone data and baseline fertility characteristics of GNRHR p.Q106R heterozygotes are comparable to those of homozygous wild-type individuals who have no reproductive problems. The heterozygous genotype alone does not impair the levels of investigated gonadotropins and sex steroid hormones or affect fertility. GNRHR p.Q106R heterozygotes who exhibit IHH characteristics must have at least another variant, probably in a different IHH gene, that drives pathogenicity. We also conclude that GNRHR p.Q106R is likely a founder variant due to its overrepresentation and prevalence in the island population of Malta.
Keywords: GNRHR founder effect; GnRH deficiency; hormone profiles; idiopathic hypogonadotropic hypogonadism; infertility; puberty.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Similar articles
-
Molecular defects of the GnRH-receptor gene in Chinese patients with idiopathic hypogonadotropic hypogonadism and the severity of hypogonadism.J Pediatr Endocrinol Metab. 2012;25(7-8):659-68. doi: 10.1515/jpem-2012-0087. J Pediatr Endocrinol Metab. 2012. PMID: 23155690
-
Role of gonadotropin-releasing hormone receptor mutations in patients with a wide spectrum of pubertal delay.Fertil Steril. 2014 Sep;102(3):838-846.e2. doi: 10.1016/j.fertnstert.2014.05.044. Epub 2014 Jul 10. Fertil Steril. 2014. PMID: 25016926 Free PMC article.
-
A new compound heterozygous mutation of the gonadotropin-releasing hormone receptor (L314X, Q106R) in a woman with complete hypogonadotropic hypogonadism: chronic estrogen administration amplifies the gonadotropin defect.J Clin Endocrinol Metab. 2000 Sep;85(9):3002-8. doi: 10.1210/jcem.85.9.6783. J Clin Endocrinol Metab. 2000. PMID: 10999776
-
Gonadotropin-Releasing Hormone Receptor (GnRHR) and Hypogonadotropic Hypogonadism.Int J Mol Sci. 2023 Nov 4;24(21):15965. doi: 10.3390/ijms242115965. Int J Mol Sci. 2023. PMID: 37958948 Free PMC article. Review.
-
Genotype and phenotype of patients with gonadotropin-releasing hormone receptor mutations.Front Horm Res. 2010;39:94-110. doi: 10.1159/000312696. Epub 2010 Apr 8. Front Horm Res. 2010. PMID: 20389088 Free PMC article. Review.
References
-
- Amato LGL, Montenegro LR, Lerario AM, et al. New genetic findings in a large cohort of congenital hypogonadotropic hypogonadism. Eur J Endocrinol. 2019;181(2):103‐119. - PubMed
-
- Kim JH, Seo GH, Kim GH, et al. Targeted gene panel sequencing for molecular diagnosis of kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Exp Clin Endocrinol Diabetes. 2019;127(8):538‐544. - PubMed
LinkOut - more resources
Full Text Sources
