A BEAT-PCD consensus statement: a core outcome set for pulmonary disease interventions in primary ciliary dyskinesia
- PMID: 38196895
- PMCID: PMC10772902
- DOI: 10.1183/23120541.00115-2023
A BEAT-PCD consensus statement: a core outcome set for pulmonary disease interventions in primary ciliary dyskinesia
Abstract
Background: Consistent use of reliable and clinically appropriate outcome measures is a priority for clinical trials, with clear definitions to allow comparability. We aimed to develop a core outcome set (COS) for pulmonary disease interventions in primary ciliary dyskinesia (PCD).
Methods: A multidisciplinary international PCD expert panel was set up. A list of outcomes was created based on published literature. Using a modified three-round e-Delphi technique, the panel was asked to decide on relevant end-points related to pulmonary disease interventions and how they should be reported. First, inclusion of an outcome in the COS was determined. Second, the minimum information that should be reported per outcome. The third round finalised statements. Consensus was defined as ≥80% agreement among experts.
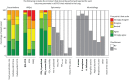
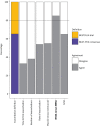
Results: During the first round, experts reached consensus on four out of 24 outcomes to be included in the COS. Five additional outcomes were discussed in subsequent rounds for their use in different subsettings. Consensus on standardised methods of reporting for the COS was reached. Spirometry, health-related quality-of-life scores, microbiology and exacerbations were included in the final COS.
Conclusion: This expert consensus resulted in a COS for clinical trials on pulmonary health among people with PCD.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: S. Aliberti has received fees outside of this work from Insmed, Zambon, AstraZeneca, CSL Behring GmbH, Grifols, Fondazione Internazionale MENARINI, MSD Italia S.r.l., Brahms, Physioassist SAS and GlaxoSmithKline. S.D. Dell has received grants outside of this work from Boehringer Ingelheim, Vertex and Sanofi, and she owns the copyright to the PCD-QOL Questionnaires. T.W. Ferkol has received consulting fees from Translate Bio and Arrowhead Pharmaceuticals. R.A. Athanazio has received personal fees outside of this work from Astrazeneca, Chiesi, GSK, Omron, Sanofi, Vertex and Zambon. K.G. Nielsen is part of the European Reference Network on respiratory diseases (ERN-LUNG) and director of PCD CTN. F.C. Ringshauen has received fees outside of this work from AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon. M. Shteinberg has received consulting fees from AstraZeneca, Boehringer Ingelheim, Dexcel, Kamada, Synchrony Medical, Trumed and Zambon. J.D. Chalmers has received grants outside of this work from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Gilead Sciences, Grifols, Insmed, Janssen, Novartis, Pfizer and Zambon; and is an associate editor of this journal. The remaining authors have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
