Transcription factor Tcf21 modulates urinary bladder size and differentiation
- PMID: 38197329
- PMCID: PMC11457511
- DOI: 10.1111/dgd.12906
Transcription factor Tcf21 modulates urinary bladder size and differentiation
Abstract
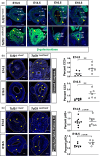
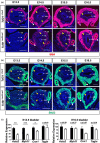
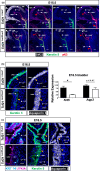
Urinary bladder organogenesis requires coordinated cell growth, specification, and patterning of both mesenchymal and epithelial compartments. Tcf21, a gene that encodes a helix-loop-helix transcription factor, is specifically expressed in the mesenchyme of the bladder during development. Here we show that Tcf21 is required for normal development of the bladder. We found that the bladders of mice lacking Tcf21 were notably hypoplastic and that the Tcf21 mutant mesenchyme showed increased apoptosis. There was also a marked delay in the formation of visceral smooth muscle, accompanied by a defect in myocardin (Myocd) expression. Interestingly, there was also a marked delay in the formation of the basal cell layer of the urothelium, distinguished by diminished expression of Krt5 and Krt14. Our findings suggest that Tcf21 regulates the survival and differentiation of mesenchyme cell-autonomously and the maturation of the adjacent urothelium non-cell-autonomously during bladder development.
Keywords: bladder; development; smooth muscle; transcription factor Tcf21; urothelium.
© 2024 The Authors. Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures


Similar articles
-
Coronary Disease-Associated Gene TCF21 Inhibits Smooth Muscle Cell Differentiation by Blocking the Myocardin-Serum Response Factor Pathway.Circ Res. 2020 Feb 14;126(4):517-529. doi: 10.1161/CIRCRESAHA.119.315968. Epub 2019 Dec 9. Circ Res. 2020. PMID: 31815603 Free PMC article.
-
Transcription Factor 21 Is Required for Branching Morphogenesis and Regulates the Gdnf-Axis in Kidney Development.J Am Soc Nephrol. 2018 Dec;29(12):2795-2808. doi: 10.1681/ASN.2017121278. Epub 2018 Oct 30. J Am Soc Nephrol. 2018. PMID: 30377232 Free PMC article.
-
TCF21: a critical transcription factor in health and cancer.J Mol Med (Berl). 2020 Aug;98(8):1055-1068. doi: 10.1007/s00109-020-01934-7. Epub 2020 Jun 15. J Mol Med (Berl). 2020. PMID: 32542449 Review.
-
Stromal Transcription Factor 21 Regulates Development of the Renal Stroma via Interaction with Wnt/β-Catenin Signaling.Kidney360. 2022 May 6;3(7):1228-1241. doi: 10.34067/KID.0005572021. eCollection 2022 Jul 28. Kidney360. 2022. PMID: 35919523 Free PMC article.
-
Smooth muscle differentiation and patterning in the urinary bladder.Differentiation. 2010 Sep-Oct;80(2-3):106-17. doi: 10.1016/j.diff.2010.05.004. Epub 2010 Jun 12. Differentiation. 2010. PMID: 20541860 Free PMC article. Review.
References
-
- Acharya, A. , Baek, S. T. , Huang, G. , Eskiocak, B. , Goetsch, S. , Sung, C. Y. , Banfi, S. , Sauer, M. F. , Olsen, G. S. , Duffield, J. S. , Olson, E. N. , & Tallquist, M. D. (2012). The bHLH transcription factor Tcf21 is required for lineage‐specific EMT of cardiac fibroblast progenitors. Development, 139(12), 2139–2149. 10.1242/dev.079970 - DOI - PMC - PubMed
-
- Braitsch, C. M. , Combs, M. D. , Quaggin, S. E. , & Yutzey, K. E. (2012). Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium‐derived cells into smooth muscle in the developing heart. Developmental Biology, 368(2), 345–357. 10.1016/j.ydbio.2012.06.002 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

