Oral killed cholera vaccines for preventing cholera
- PMID: 38197546
- PMCID: PMC10777452
- DOI: 10.1002/14651858.CD014573
Oral killed cholera vaccines for preventing cholera
Abstract
Background: Cholera causes acute watery diarrhoea and death if not properly treated. Outbreaks occur in areas with poor sanitation, including refugee camps. Several vaccines have been developed and tested over the last 50 years. This is an update of a Cochrane review, originally published in 1998, which explored the effects of all vaccines for preventing cholera. This review examines oral vaccines made from killed bacteria.
Objectives: To assess the effectiveness and safety of the available World Health Organization (WHO)-prequalified oral killed cholera vaccines among children and adults.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; CENTRAL, MEDLINE; Embase; LILACS; and two trials registers (February 2023).
Selection criteria: We included randomized controlled trials (RCTs), including cluster-RCTs. There were no restrictions on the age and sex of the participants or the setting of the study. We considered any available WHO-prequalified oral killed cholera vaccine as an intervention. The control group was given a placebo, another vaccine, or no vaccine. The outcomes were related to vaccine effectiveness and safety. We included articles published in English only.
Data collection and analysis: Two review authors independently applied the inclusion criteria and extracted data from included studies. We assessed the risk of bias using the Cochrane ROB 1 assessment tool. We used the generic inverse variance and a random-effects model meta-analysis to estimate the pooled effect of the interventions. We assessed the certainty of the evidence using the GRADE approach. For vaccine effectiveness (VE), we converted the overall risk ratio (RR) to vaccine effectiveness using the formula: VE = (1 - RR) x 100%.
Main results: Five RCTs, reported in 12 records, with 462,754 participants, met the inclusion criteria. We identified trials on whole-cell plus recombinant vaccine (WC-rBS vaccine (Dukoral)) from Peru and trials on bivalent whole-cell vaccine (BivWC (Shanchol)) vaccine from India and Bangladesh. We did not identify any trials on other BivWC vaccines (Euvichol/Euvichol-Plus), or Hillchol. Two doses of Dukoral with or without a booster dose reduces cases of cholera at two-year follow-up in a general population of children and adults, and at five-month follow-up in an adult male population (overall VE 76%; RR 0.24, 95% confidence interval (CI) 0.08 to 0.65; 2 trials, 16,423 participants; high-certainty evidence). Two doses of Shanchol reduces cases of cholera at one-year follow-up (overall VE 37%; RR 0.63, 95% CI 0.47 to 0.85; 2 trials, 241,631 participants; high-certainty evidence), at two-year follow-up (overall VE 64%; RR 0.36, 95% CI 0.16 to 0.81; 2 trials, 168,540 participants; moderate-certainty evidence), and at five-year follow-up (overall VE 80%; RR 0.20, 95% CI 0.15 to 0.26; 1 trial, 54,519 participants; high-certainty evidence). A single dose of Shanchol reduces cases of cholera at six-month follow-up (overall VE 40%; RR 0.60, 95% CI 0.47 to 0.77; 1 trial, 204,700 participants; high-certainty evidence), and at two-year follow-up (overall VE 39%; RR 0.61, 95% CI 0.53 to 0.70; 1 trial, 204,700 participants; high-certainty evidence). A single dose of Shanchol also reduces cases of severe dehydrating cholera at six-month follow-up (overall VE 63%; RR 0.37, 95% CI 0.28 to 0.50; 1 trial, 204,700 participants; high-certainty evidence), and at two-year follow-up (overall VE 50%; RR 0.50, 95% CI 0.42 to 0.60; 1 trial, 204,700 participants; high-certainty evidence). We found no differences in the reporting of adverse events due to vaccination between the vaccine and control/placebo groups.
Authors' conclusions: Two doses of Dukoral reduces cases of cholera at two-year follow-up. Two doses of Shanchol reduces cases of cholera at five-year follow-up, and a single dose of Shanchol reduces cases of cholera at two-year follow-up. Overall, the vaccines were safe and well-tolerated. We found no trials on other BivWC vaccines (Euvichol/Euvichol-Plus). However, BivWC products (Shanchol, Euvichol/Euvichol-Plus) are considered to produce comparable vibriocidal responses. Therefore, it is reasonable to apply the results from Shanchol trials to the other BivWC products (Euvichol/Euvichol-Plus).
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
KM Saif‐Ur‐Rahman: none declared
Razib Mamun: none declared
Md Hasan: none declared
James Meiring: none declared
Arifuzzaman Khan is a co‐author in two included articles (Qadri 2015; Qadri 2016). He was not engaged in data extraction or assessment of the risk of bias of the included articles.
Figures
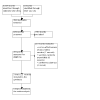
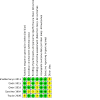
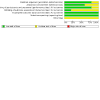









References
References to studies included in this review
Bhattacharya 2013 {published data only}
-
- Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, et al. 5-year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infectious Diseases 2013;13(12):1050-6. [PMID: 10.1016/S1473-3099(13)70273-1] - DOI - PubMed
-
- Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 2009;374(9702):1694‐702. - PubMed
Qadri 2015 {published data only}
Qadri 2016 {published data only}
-
- Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, et al. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infectious Diseases 2018;18(6):666-74. [PMID: 10.1016/S1473-3099(18)30108-7] - DOI - PubMed
Sanchez 1994 {published data only}
Taylor 2000 {published data only}
References to studies excluded from this review
Baik 2014 {published data only}
Chowdhury 2022 {published data only}
Clemens 1986 {published data only}
-
- Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, et al. Field trial of oral cholera vaccines in Bangladesh. Southeast Asian Journal of Tropical Medicine and Public Health 1988 ;19(3):417-22. [PMID: PMID: 3217823] - PubMed
-
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, et al. Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet 1988;1(8599):1375-9. [DOI: 10.1016/s0140-6736(88)92189-7] - DOI - PubMed
Hashim 2012 {published data only}
Khan 2016 {published data only}
-
- Khan AI, Ali M, Kabir A, Chowdhury F, Saha A, Khan IA, et al. Effect of a bivalent, killed, whole cell oral cholera vaccine on pregnancy outcome in Bangladesh. American Journal of Tropical Medicine and Hygiene 2016;95(5 Suppl 1):324‐5.
Mahalanabis 2008 {published data only}
-
- Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, et al. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One 2008 ;3(6):e2323. [DOI: 10.1371/journal.pone.0002323] - DOI - PMC - PubMed
Mwaba 2021 {published data only}
Russo 2018 {published data only}
-
- Russo P, Ligsay AD, Olveda R, Choi SK, Kim DR, Park JY, et al. A randomized, observer-blinded, equivalence trial comparing two variations of Euvichol®, a bivalent killed whole-cell oral cholera vaccine, in healthy adults and children in the Philippines. Vaccine 2018 ;36(29):4317-24. [DOI: 10.1016/j.vaccine.2018.05.102] - DOI - PMC - PubMed
Savarino 2002 {published data only}
-
- Savarino SJ, Hall ER, Bassily S, Wierzba TF, Youssef FG, Peruski LF Jr, et al. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. The Pediatric Infectious Disease Journal 2002 ;21(4):322-30. [DOI: 10.1097/00006454-200204000-00012] - DOI - PubMed
Additional references
Bhadra 1994
-
- Bhadra RK, Dasgupta U, Das J. Cholera vaccine: developmental strategies and problems. Indian Journal of Biochemistry and Biophysics 1994;31(6):441-8. - PubMed
Bi 2017
-
- Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, et al, Oral Cholera Vaccine Working Group of the Global Task Force on Cholera Control. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infectious Diseases 2017;17(10):1080-8. - PMC - PubMed
Chowdhury 2021
-
- Chowdhury F, Ali Syed K, Akter A, Rahman Bhuiyan T, Tauheed I, Khaton F, et al. A phase I/II study to evaluate safety, tolerability and immunogenicity of Hillchol®, an inactivated single Hikojima strain based oral cholera vaccine, in a sequentially age descending population in Bangladesh. Vaccine 2021;39(32):4450-7. [PMID: ] - PubMed
Clemens 1989
-
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Huda S, et al. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. Journal of Infectious Diseases 1989;159(4):770-3. - PubMed
Finkelstein 1996
-
- Finkelstein RA. Chapter 24: Cholera, Vibrio cholerae O1 and O139, and other pathogenic Vibrios. In: Baron S, editors(s). Medical Microbiology. 4th edition. Vol. 24. Galveston (TX): University of Texas Medical Branch at Galveston, 1996. - PubMed
Girard 2006
-
- Girard MP, Steele D, Chaignat CL, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine 2006;24(15):2732-50. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 1 August 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harris 2005
Harris 2016
Heymann 2008
-
- Heyman DL. Control of Communicable Diseases Manual. 19th edition. Washington (DC): American Public Health Association, 2008.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Hill 2006
-
- Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infectious Diseases 2006;6(6):361-73. - PubMed
Holmgren 2005
-
- Holmgren J, Adamsson J, Anjuère F, Clemens J, Czerkinsky C, Eriksson K, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunology Letters 2005;97(2):181-8. - PubMed
Kanungo 2015
Lopez 2014
Lopez 2018
-
- Lopez AL, Deen J, Azman AS, Luquero FJ, Kanungo S, Dutta S, et al. Immunogenicity and protection from a single dose of internationally available killed oral cholera vaccine: a systematic review and metaanalysis. Clinical Infectious Diseases 2018;66(12):1960-71. [PMID: 10.1093/cid/cix1039] - DOI - PMC - PubMed
Panda 2020
Ramamurthy 2010
-
- Ramamurthy T, Wagener D, Chowdhury G, Majumder PP. A large study on immunological response to a whole-cell killed oral cholera vaccine reveals that there are significant geographical differences in response and that O blood group individuals do not elicit a higher response. Clinical and Vaccine Immunology 2010;17(8):1232-7. - PMC - PubMed
RevMan Web 2023 [Computer program]
-
- Review Manager Web (RevMan Web). Version 6.7.1. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Sack 2004
-
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet 2004;363(9404):223-33. - PubMed
Schwerdtle 2018
-
- Schwerdtle P, Onekon CK, Recoche K. A quantitative systematic review and meta-analysis of the effectiveness of oral cholera vaccine as a reactive measure in cholera outbreaks. Prehospital and Disaster Medicine 2018;33(1):2-6. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sousa 2020
Sánchez 1997
-
- Sánchez JL, Taylor DN. Cholera. Lancet 1997;349(9068):1825-30. - PubMed
Teoh 2018
WHO 2006
-
- World Health Organization (WHO). Cholera 2005. Weekly Epidemiological Record 2006;81(31):297-307.
WHO 2009
-
- World Health Organization (WHO). Outbreak news: cholera, Zimbabwe - update. Weekly Epidemiological Record 2009;84(14):109-10.
WHO 2010
-
- World Health Organization (WHO). Outbreak news: cholera, Haiti – update. Weekly Epidemiological Record 2010;85(49):489-90.
WHO 2017
-
- World Health Organization (WHO). Cholera vaccines: WHO position paper – August 2017. Weekly Epidemiological Record 2017;92(34):477-500. [PMID: ] - PubMed
WHO 2022
-
- World Health Organization (WHO). Cholera. who.int/news-room/fact-sheets/detail/cholera (accessed 10 May 2023).
WHO 2023
-
- World Health Organization (WHO). Cholera – Global situation. who.int/emergencies/disease-outbreak-news/item/2023-DON437 (accessed 10 May 2023).
References to other published versions of this review
Graves 2001
Graves 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

