Epicardial adipose tissue dispersion at CT and recurrent atrial fibrillation after pulmonary vein isolation
- PMID: 38197916
- PMCID: PMC11255050
- DOI: 10.1007/s00330-023-10498-2
Epicardial adipose tissue dispersion at CT and recurrent atrial fibrillation after pulmonary vein isolation
Abstract
Objectives: Epicardial adipose tissue (EAT) remodeling is associated with atrial fibrillation (AF). Left atrial (LA) EAT dispersion on cardiac CT is a non-invasive imaging biomarker reflecting EAT heterogeneity. We aimed to investigate the association of LA EAT dispersion with AF recurrence after pulmonary vein isolation (PVI).
Methods: In a prospective registry of consecutive patients undergoing first PVI, mean EAT attenuation values were measured on contrast-enhanced cardiac CT scans in Hounsfield units (HU) within low (- 195 to - 45 HU) and high (- 44 to - 15 HU) threshold EAT compartments around the left atrium (LA). EAT dispersion was defined as the difference between the mean HU values within the two EAT compartments. Continuous variables were compared between groups using the Mann-Whitney U test and cox proportional hazard models were used to calculate hazard ratios of predictors of 1-year AF recurrence.
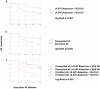
Results: A total of 208 patients were included, 135 with paroxysmal AF and 73 with persistent AF. LA EAT dispersion was significantly larger in patients with persistent compared to paroxysmal AF (52.6 HU vs. 49.9 HU; p = 0.001). After 1 year of follow-up, LA EAT dispersion above the mean (> 50.8 HU) was associated with a higher risk of AF recurrence (HR 2.3, 95% CI 1.5-3.6; p < 0.001). It retained its predictive value when corrected for age, sex, body mass index, LA volume, and AF type (HR 2.8, 95% CI 1.6-4.6; p < 0.001).
Conclusion: A larger LA EAT dispersion on contrast-enhanced cardiac CT scans, reflecting EAT heterogeneity, is independently associated with AF recurrence after PVI.
Clinical relevance statement: Based on LA EAT dispersion assessment, a more accurate risk stratification and patient selection may be possible based on a pre-procedural cardiac CT when planning PVI.
Key points: • Epicardial adipose tissue (EAT) remodeling is associated with atrial fibrillation (AF). • A larger left atrial EAT dispersion in a pre-procedural cardiac CT was associated with a higher 1-year AF recurrence risk after pulmonary vein isolation. • A pre-procedural cardiac CT with left atrial EAT dispersion assessment may provide a more accurate risk stratification and patient selection for PVI.
Keywords: Adipose tissue; Atrial fibrillation; Catheter ablation; Coronary vessels; Multidetector computed tomography.
© 2024. The Author(s).
Conflict of interest statement
Dr. Reichlin has received research grants from the Goldschmidt-Jacobson Foundation, the Swiss National Science Foundation, the Swiss Heart Foundation, and the sitem-insel support fund, all for work outside the submitted study. He has received speaker/consulting honoraria or travel support from Abbott/SJM, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Farapulse, Medtronic, and Pfizer-BMS, all for work outside the submitted study. He has received support for his institution’s fellowship program from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific, and Medtronic for work outside the submitted study.
Dr. Huber has received research grants from the Swiss National Science Foundation, the Helmut-Hartweg Foundation, and the Foundation to Fight against Cancer, all for work outside the submitted study. He has received speaker/consulting honoraria or travel support from Bayer, Bracco, and Siemens, all for work outside the submitted study.
Dr. Tanner reports educational grants from Biosense-Webster and travel grants from Abbott.
Dr. Haeberlin: travel/educational grants from Medtronic and Philips/Spectranetics; co-founder and head of Act-Inno, a cardiovascular device testing company; research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the Swiss Heart Rhythm Foundation, the Swiss Pacemaker Foundation, the Hasler Foundation, the Velux Foundation, Novartis, and the sitem-insel Support Funds, all for work outside the submitted study; consultant/advisor for DiNAQOR and Biotronik for work outside the submitted study.
Dr. Gräni received funding from the Swiss National Science Foundation, InnoSuisse, the Center of Artificial Intelligence University of Bern, and the GMABIT foundation, outside of the submitted work.
Dr. Roten received research grants from Medtronic and speaker/consulting honoraria from Abbott and Medtronic.
Dr. von Tengg-Kobligk received funding from the Swiss National Science Foundation and InnoSuisse. He has received speaker/consulting honoraria from Mint Medical, all outside of the submitted work.
All other authors report no conflicts of interest related to this paper.
Figures


References
-
- Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018;15(11):1717–1727. doi: 10.1016/j.hrthm.2018.06.025. - DOI - PubMed
-
- Franssens BT, Nathoe HM, Visseren FLJ, van der Graaf Y, Leiner T, Algra A, et al. Relation of epicardial adipose tissue radiodensity to coronary artery calcium on cardiac computed tomography in patients at high risk for cardiovascular disease. Am J Cardiol. 2017;119(9):1359–65. doi: 10.1016/j.amjcard.2017.01.031. - DOI - PubMed

