Prophylactic maintenance with venetoclax/azacitidine after reduced-intensity conditioning allogeneic transplant for high-risk MDS and AML
- PMID: 38197938
- PMCID: PMC10883823
- DOI: 10.1182/bloodadvances.2023012120
Prophylactic maintenance with venetoclax/azacitidine after reduced-intensity conditioning allogeneic transplant for high-risk MDS and AML
Abstract
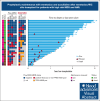
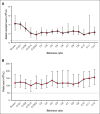
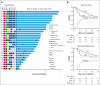
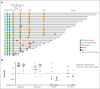
We conducted a phase 1 trial assessing safety and efficacy of prophylactic maintenance therapy with venetoclax and azacitidine (Ven/Aza) for patients with high-risk myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML) undergoing reduced intensity allogeneic stem cell transplantation (allo-SCT) after Ven and fludarabine/busulfan conditioning (Ven/FluBu2 allo-SCT) with tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis. Among 27 patients who underwent Ven/FluBu2 allo-SCT (55.6% with prior Ven exposure, and 96% with positive molecular measurable residual disease), 22 received maintenance therapy with Aza 36 mg/m2 intravenously on days 1 to 5, and Ven 400 mg by mouth on days 1 to 14 per assigned dose schedule/level (42-day cycles × 8, or 28-day cycles × 12). During maintenance, the most common grade 3-4 adverse events were leukopenia, neutropenia, and thrombocytopenia, which were transient and manageable. Infections were uncommon (n = 4, all grade 1-2). The 1-year and 2-year moderate/severe chronic GVHD rates were 4% (95% confidence interval [CI], 0.3%-18%) and 22% (95% CI, 9%-40%), respectively. After a median follow-up of 25 months among survivors, the median overall survival (OS) was not reached. Among the 22 patients who received Ven/Aza maintenance, the 2-year OS, progression-free survival, nonrelapse mortality, and cumulative incidence of relapse rates were 67% (95% CI, 43%-83%), 59% (95% CI, 36%-76%), 0%, and 41% (95% CI, 20%-61%), respectively. Immune monitoring demonstrated no significant impact on T-cell expansion but identified reduced B-cell expansion compared with controls. This study demonstrates prophylactic Ven/Aza maintenance can be safely administered for patients with high-risk MDS/AML, but a randomized study is required to properly assess any potential benefit. This trial was registered at www.clinicaltrials.gov as #NCT03613532.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.S.G. reports grants from and serves on advisory boards for AbbVie, Astellas, Bristol Myers Squibb, Genentech, and Servier, and receives grants/research funding from AbbVie, Genentech, New Wave, and Pfizer. C.S.C. serves on the advisory board/consults for Sanofi, InhibRx, Cellarity, Astellas, Rigel, Novartis, Incyte, Cimeio, and Oxford Immune Algorithmics, and serves on the data and safety monitoring board for AlloVir and Angiocrine. J.K. serves on board and consults for Mallinckrodt, EMD Serono, Merck, Cugene, Cue Biotherapeutics, Biolojic Design, Gentibio, Nekonal, Equillium, and Amgen, and receives grants/research support from Bristol Myers Squibb, Miltenyi, Regeneron, Equillium, Amgen and Clinigen. R.R. receives grant support from CRISPR Therapeutics and serves on the advisory board for Glycostem. D.J.D. consults for AbbVie, Amgen, Agios, Autolus, Blueprint, Forty-Seven, Glycomimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda. R.M.S. reports grants and personal fees from AbbVie, Agios, and Novartis; personal fees from Actinium, Argenx, Astellas, AstraZeneca, BioLineRx, Celgene, Daiichi Sankyo, Elevate, GEMoaB, Janssen, Jazz, MacroGenics, Otsuka, Pfizer, Hoffmann–La Roche, Stemline, Syndax, Syntrix, Syros, Takeda, and Trovagene; and grants from Arog. J.H.A. serves on the data and safety monitoring board for CSL Behring and Janssen, and serves on the scientific advisory board for Pharmacosmos. J.R. receives research support from Equillium, Kite/Gilead, Novartis, and Oncternal Therapeutics, and serves on advisory boards for Akron Biotech, Clade Therapeutics, Garuda Therapeutics, LifeVault Bio, Novartis, Smart Immune, and TScan Therapeutics. A.L. serves on the scientific advisory board of Flash Therapeutics, Trueline Therapeutics, and Zentalis Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures

References
-
- Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–1377. - PubMed
-
- Devine SM, Owzar K, Blum W, et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from cancer and leukemia group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33(35):4167–4175. - PMC - PubMed
-
- Ustun C, Le-Rademacher J, Wang HL, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33(11):2599–2609. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

