Electro-metabolic signaling
- PMID: 38197953
- PMCID: PMC10783436
- DOI: 10.1085/jgp.202313451
Electro-metabolic signaling
Abstract
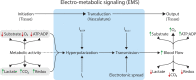
Precise matching of energy substrate delivery to local metabolic needs is essential for the health and function of all tissues. Here, we outline a mechanistic framework for understanding this critical process, which we refer to as electro-metabolic signaling (EMS). All tissues exhibit changes in metabolism over varying spatiotemporal scales and have widely varying energetic needs and reserves. We propose that across tissues, common signatures of elevated metabolism or increases in energy substrate usage that exceed key local thresholds rapidly engage mechanisms that generate hyperpolarizing electrical signals in capillaries that then relax contractile elements throughout the vasculature to quickly adjust blood flow to meet changing needs. The attendant increase in energy substrate delivery serves to meet local metabolic requirements and thus avoids a mismatch in supply and demand and prevents metabolic stress. We discuss in detail key examples of EMS that our laboratories have discovered in the brain and the heart, and we outline potential further EMS mechanisms operating in tissues such as skeletal muscle, pancreas, and kidney. We suggest that the energy imbalance evoked by EMS uncoupling may be central to cellular dysfunction from which the hallmarks of aging and metabolic diseases emerge and may lead to generalized organ failure states-such as diverse flavors of heart failure and dementia. Understanding and manipulating EMS may be key to preventing or reversing these dysfunctions.
© 2024 Longden and Lederer.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures




References
-
- Abd El-Rahman, R.R., Harraz O.F., Brett S.E., Anfinogenova Y., Mufti R.E., Goldman D., and Welsh D.G.. 2013. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. Am. J. Physiol. Heart Circ. Physiol. 304:H58–H71. 10.1152/ajpheart.00476.2012 - DOI - PMC - PubMed
-
- Ahn, S.J., Le Master E., Lee J.C., Phillips S.A., Levitan I., and Fancher I.S.. 2022. Differential effects of obesity on visceral versus subcutaneous adipose arteries: Role of shear-activated Kir2.1 and alterations to the glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 322:H156–H166. 10.1152/ajpheart.00399.2021 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

