Real-World Experience of Carglumic Acid for Methylmalonic and Propionic Acidurias: An Interim Analysis of the Multicentre Observational PROTECT Study
- PMID: 38198106
- PMCID: PMC11035519
- DOI: 10.1007/s40268-023-00449-z
Real-World Experience of Carglumic Acid for Methylmalonic and Propionic Acidurias: An Interim Analysis of the Multicentre Observational PROTECT Study
Abstract
Background and objective: Methylmalonic aciduria (MMA) and propionic aciduria (PA) are organic acidurias characterised by the accumulation of toxic metabolites and hyperammonaemia related to secondary N-acetylglutamate deficiency. Carglumic acid, a synthetic analogue of N-acetylglutamate, decreases ammonia levels by restoring the functioning of the urea cycle. However, there are limited data available on the long-term safety and effectiveness of carglumic acid. Here, we present an interim analysis of the ongoing, long-term, prospective, observational PROTECT study (NCT04176523), which is investigating the long-term use of carglumic acid in children and adults with MMA and PA.
Methods: Individuals with MMA or PA from France, Germany, Italy, Norway, Spain, Sweden and the UK who have received at least 1 year of carglumic acid treatment as part of their usual care are eligible for inclusion. The primary objective is the number and duration of acute metabolic decompensation events with hyperammonaemia (ammonia level >159 µmol/L during a patient's first month of life or >60 µmol/L thereafter, with an increased lactate level [> 1.8 mmol/L] and/or acidosis [pH < 7.35]) before and after treatment with carglumic acid. Peak plasma ammonia levels during the last decompensation event before and the first decompensation event after carglumic acid initiation, and the annualised rate of decompensation events before and after treatment initiation are also being assessed. Secondary objectives include the duration of hospital stay associated with decompensation events. Data are being collected at approximately 12 months' and 18 months' follow-up.
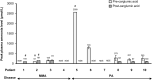
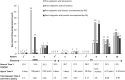
Results: Of the patients currently enrolled in the PROTECT study, data from ten available patients with MMA (n = 4) and PA (n = 6) were analysed. The patients had received carglumic acid for 14-77 (mean 36) months. Carglumic acid reduced the median peak ammonia level of the total patient population from 250 µmol/L (range 97-2569) before treatment to 103 µmol/L (range 97-171) after treatment. The annualised rate of acute metabolic decompensations with hyperammonaemia was reduced by a median of - 41% (range - 100% to + 60%) after treatment with carglumic acid. Of the five patients who experienced a decompensation event before treatment and for whom a post-treatment rate could be calculated, the annualised decompensation event rate was lower after carglumic acid treatment in four patients. The mean duration of hospital inpatient stay during decompensation events was shorter after than before carglumic acid treatment initiation in four of five patients for whom length of stay could be calculated.
Conclusions: In this group of patients with MMA and PA, treatment with carglumic acid for at least 1 year reduced peak plasma ammonia levels in the total patient population and reduced the frequency of metabolic decompensation events, as well as the duration of inpatient stay due to metabolic decompensations in a subset of patients.
Clinical trial registration: ClinicalTrials.gov, NCT04176523. Registered 25 November, 2019, retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT04176523 .
© 2024. Crown.
Conflict of interest statement
Sufin Yap has received funding for conferences and honorarium for lectures, master classes, advisory boards and ‘meet the expert’ sessions from Recordati Rare Diseases; and honorarium for expert advice from OpenVie. Vincenzo Giordano is an employee of Recordati Rare Diseases. Delphine Lamireau, Francois Feillet, Angeles Ruiz Gomez, James Davison, and Trine Tangeraas have no conflicts of interest that are directly relevant to the content of this article.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

