Endeavor toward Redox-Responsive Transition Metal Contrast Agents Based on the Cross-Bridge Cyclam Platform
- PMID: 38198518
- PMCID: PMC10806912
- DOI: 10.1021/acs.inorgchem.3c03486
Endeavor toward Redox-Responsive Transition Metal Contrast Agents Based on the Cross-Bridge Cyclam Platform
Abstract
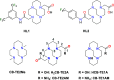
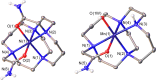
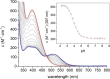
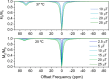
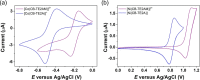
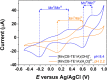
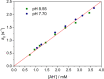
We present the synthesis and characterization of a series of Mn(III), Co(III), and Ni(II) complexes with cross-bridge cyclam derivatives (CB-cyclam = 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane) containing acetamide or acetic acid pendant arms. The X-ray structures of [Ni(CB-TE2AM)]Cl2·2H2O and [Mn(CB-TE1AM)(OH)](PF6)2 evidence the octahedral coordination of the ligands around the Ni(II) and Mn(III) metal ions, with a terminal hydroxide ligand being coordinated to Mn(III). Cyclic voltammetry studies on solutions of the [Mn(CB-TE1AM)(OH)]2+ and [Mn(CB-TE1A)(OH)]+ complexes (0.15 M NaCl) show an intricate redox behavior with waves due to the MnIII/MnIV and MnII/MnIII pairs. The Co(III) and Ni(II) complexes with CB-TE2A and CB-TE2AM show quasi-reversible features due to the CoIII/CoII or NiII/NiIII pairs. The [Co(CB-TE2AM)]3+ complex is readily reduced by dithionite in aqueous solution, as evidenced by 1H NMR studies, but does not react with ascorbate. The [Mn(CB-TE1A)(OH)]+ complex is however reduced very quickly by ascorbate following a simple kinetic scheme (k0 = k1[AH-], where [AH-] is the ascorbate concentration and k1 = 628 ± 7 M-1 s-1). The reduction of the Mn(III) complex to Mn(II) by ascorbate provokes complex dissociation, as demonstrated by 1H nuclear magnetic relaxation dispersion studies. The [Ni(CB-TE2AM)]2+ complex shows significant chemical exchange saturation transfer effects upon saturation of the amide proton signals at 71 and 3 ppm with respect to the bulk water signal.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Synthesis, characterization, and ligand exchange reactivity of a series of first row divalent metal 3-hydroxyflavonolate complexes.Inorg Chem. 2010 Jan 4;49(1):82-96. doi: 10.1021/ic901405h. Inorg Chem. 2010. PMID: 19954165
-
Seven coordinate Co(ii) and six coordinate Ni(ii) complexes of an aromatic macrocyclic triamide ligand as paraCEST agents for MRI.Dalton Trans. 2019 Jun 28;48(24):8899-8910. doi: 10.1039/c9dt00747d. Epub 2019 May 29. Dalton Trans. 2019. PMID: 31140528
-
Molecular and electronic structures of bis(pyridine-2,6-diimine)metal complexes [ML2](PF6)n (n = 0, 1, 2, 3; M = Mn, Fe, Co, Ni, Cu, Zn).Inorg Chem. 2000 Jun 26;39(13):2936-47. doi: 10.1021/ic000113j. Inorg Chem. 2000. PMID: 11232835
-
Mn2+ complexes with 12-membered pyridine based macrocycles bearing carboxylate or phosphonate pendant arm: crystallographic, thermodynamic, kinetic, redox, and 1H/17O relaxation studies.Inorg Chem. 2011 Dec 19;50(24):12785-801. doi: 10.1021/ic201935r. Epub 2011 Nov 17. Inorg Chem. 2011. PMID: 22092039
-
Seven-coordinate Co(II), Fe(II) and six-coordinate Ni(II) amide-appended macrocyclic complexes as ParaCEST agents in biological media.Inorg Chem. 2014 Aug 18;53(16):8311-21. doi: 10.1021/ic5006083. Epub 2014 May 13. Inorg Chem. 2014. PMID: 24820102 Free PMC article.
References
-
- Lohrke J.; Frenzel T.; Endrikat J.; Alves F. C.; Grist T. M.; Law M.; Lee J. M.; Leiner T.; Li K.-C.; Nikolaou K.; Prince M. R.; Schild H. H.; Weinreb J. C.; Yoshikawa K.; Pietsch H. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33 (1), 1–28. 10.1007/s12325-015-0275-4. - DOI - PMC - PubMed
-
- Doan B.-T.; Meme S.; Beloeil J.-C.. General Principles of MRI. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Merbach A., Helm L., Tóth E. ´., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp 1–23.
LinkOut - more resources
Full Text Sources

