Local and dynamic regulation of neuronal glycolysis in vivo
- PMID: 38198527
- PMCID: PMC10801914
- DOI: 10.1073/pnas.2314699121
Local and dynamic regulation of neuronal glycolysis in vivo
Abstract
Energy metabolism supports neuronal function. While it is well established that changes in energy metabolism underpin brain plasticity and function, less is known about how individual neurons modulate their metabolic states to meet varying energy demands. This is because most approaches used to examine metabolism in living organisms lack the resolution to visualize energy metabolism within individual circuits, cells, or subcellular regions. Here, we adapted a biosensor for glycolysis, HYlight, for use in Caenorhabditis elegans to image dynamic changes in glycolysis within individual neurons and in vivo. We determined that neurons cell-autonomously perform glycolysis and modulate glycolytic states upon energy stress. By examining glycolysis in specific neurons, we documented a neuronal energy landscape comprising three general observations: 1) glycolytic states in neurons are diverse across individual cell types; 2) for a given condition, glycolytic states within individual neurons are reproducible across animals; and 3) for varying conditions of energy stress, glycolytic states are plastic and adapt to energy demands. Through genetic analyses, we uncovered roles for regulatory enzymes and mitochondrial localization in the cellular and subcellular dynamic regulation of glycolysis. Our study demonstrates the use of a single-cell glycolytic biosensor to examine how energy metabolism is distributed across cells and coupled to dynamic states of neuronal function and uncovers unique relationships between neuronal identities and metabolic landscapes in vivo.
Keywords: C. elegans; biosensor; energy metabolism; glycolysis; neurons.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
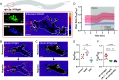
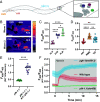
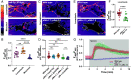
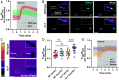
Update of
-
Local and dynamic regulation of neuronal glycolysis in vivo.bioRxiv [Preprint]. 2023 Aug 26:2023.08.25.554774. doi: 10.1101/2023.08.25.554774. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2314699121. doi: 10.1073/pnas.2314699121. PMID: 37662365 Free PMC article. Updated. Preprint.
References
-
- Fox P. T., Raichle M. E., Mintun M. A., Dence C., Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464 (1988). - PubMed
-
- Phelps M. E., et al. , Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann. Neurol. 6, 371–388 (1979). - PubMed
-
- Logothetis N. K., Pauls J., Augath M., Trinath T., Oeltermann A., Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

