mRNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical argininosuccinic aciduria
- PMID: 38198573
- PMCID: PMC7615535
- DOI: 10.1126/scitranslmed.adh1334
mRNA therapy corrects defective glutathione metabolism and restores ureagenesis in preclinical argininosuccinic aciduria
Abstract
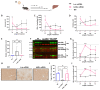
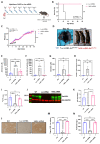
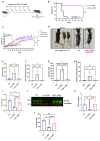
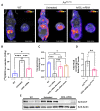
The urea cycle enzyme argininosuccinate lyase (ASL) enables the clearance of neurotoxic ammonia and the biosynthesis of arginine. Patients with ASL deficiency present with argininosuccinic aciduria, an inherited metabolic disease with hyperammonemia and a systemic phenotype coinciding with neurocognitive impairment and chronic liver disease. Here, we describe the dysregulation of glutathione biosynthesis and upstream cysteine utilization in ASL-deficient patients and mice using targeted metabolomics and in vivo positron emission tomography (PET) imaging using (S)-4-(3-18F-fluoropropyl)-l-glutamate ([18F]FSPG). Up-regulation of cysteine metabolism contrasted with glutathione depletion and down-regulated antioxidant pathways. To assess hepatic glutathione dysregulation and liver disease, we present [18F]FSPG PET as a noninvasive diagnostic tool to monitor therapeutic response in argininosuccinic aciduria. Human hASL mRNA encapsulated in lipid nanoparticles improved glutathione metabolism and chronic liver disease. In addition, hASL mRNA therapy corrected and rescued the neonatal and adult Asl-deficient mouse phenotypes, respectively, enhancing ureagenesis. These findings provide mechanistic insights in liver glutathione metabolism and support clinical translation of mRNA therapy for argininosuccinic aciduria.
Conflict of interest statement
Figures


References
-
- Baruteau J, Diez-Fernandez C, Lerner S, Ranucci G, Gissen P, Dionisi-Vici C, Nagamani S, Erez A, Häberle J. Argininosuccinic aciduria: Recent pathophysiological insights and therapeutic prospects. J Inherit Metab Dis. 2019;42:1147–1161. - PubMed
-
- Erez A. Argininosuccinic aciduria: from a monogenic to a complex disorder. Genet Med. 2013;15:251–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

