The post-stroke young adult brain has limited capacity to re-express the gene expression patterns seen during early postnatal brain development
- PMID: 38198833
- PMCID: PMC11328347
- DOI: 10.1111/bpa.13232
The post-stroke young adult brain has limited capacity to re-express the gene expression patterns seen during early postnatal brain development
Abstract
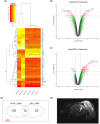
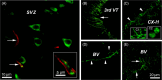
The developmental origins of the brain's response to injury can play an important role in recovery after a brain lesion. In this study, we investigated whether the ischemic young adult brain can re-express brain plasticity genes that were active during early postnatal development. Differentially expressed genes in the cortex of juvenile post-natal day 3 and the peri-infarcted cortical areas of young, 3-month-old post-stroke rats were identified using fixed-effects modeling within an empirical Bayes framework through condition-specific comparison. To further analyze potential biological processes, upregulated and downregulated genes were assessed for enrichment using GSEA software. The genes showing the highest expression changes were subsequently verified through RT-PCR. Our findings indicate that the adult brain partially recapitulates the gene expression profile observed in the juvenile brain but fails to upregulate many genes and pathways necessary for brain plasticity. Of the upregulated genes in post-stroke brains, specific roles have not been assigned to Apobec1, Cenpf, Ect2, Folr2, Glipr1, Myo1f, and Pttg1. New genes that failed to upregulate in the adult post-stroke brain include Bex4, Cd24, Klhl1/Mrp2, Trim67, and St8sia2. Among the upregulated pathways, the largest change was observed in the KEGG pathway "One carbon pool of folate," which is necessary for cellular proliferation, followed by the KEGG pathway "Antifolate resistance," whose genes mainly encode the family of ABC transporters responsible for the efflux of drugs that have entered the brain. We also noted three less-described downregulated KEGG pathways in experimental models: glycolipid biosynthesis, oxytocin, and cortisol pathways, which could be relevant as therapeutic targets. The limited brain plasticity of the adult brain is illustrated through molecular and histological analysis of the axonal growth factor, KIF4. Collectively, these results strongly suggest that further research is needed to decipher the complex genetic mechanisms that prevent the re-expression of brain plasticity-associated genes in the adult brain.
Keywords: KIF4; adult brain; axon growth; brain plasticity; juvenile brain; kinesins; stroke; transcriptomics.
© 2024 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581(7806):77–82. - PubMed
-
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

