Material properties of nonpregnant and pregnant human uterine layers
- PMID: 38198930
- PMCID: PMC11588393
- DOI: 10.1016/j.jmbbm.2023.106348
Material properties of nonpregnant and pregnant human uterine layers
Abstract
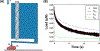
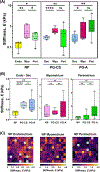
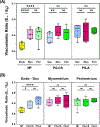
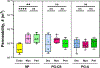
The uterus has critical biomechanical functions in pregnancy and undergoes dramatic material growth and remodeling from implantation to parturition. The intrinsic material properties of the human uterus and how they evolve in pregnancy are poorly understood. To address this knowledge gap and assess the heterogeneity of these tissues, the time-dependent material properties of all human uterine layers were measured with nanoindentation. The endometrium-decidua layer was found to be the least stiff, most viscous, and least permeable layer of the human uterus in nonpregnant and third-trimester pregnant tissues. In pregnancy, the endometrium-decidua becomes stiffer and less viscous with no material property changes observed in the myometrium or perimetrium. Additionally, uterine material properties did not significantly differ between third-trimester pregnant tissues with and without placenta accreta. The foundational data generated by this study will facilitate the development of physiologically accurate models of the human uterus to investigate gynecologic and obstetric disorders.
Keywords: Nanoindentation; Poro-viscoelasticity; Pregnancy; Reproductive biomechanics; Uterus.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Update of
-
Material Properties of Nonpregnant and Pregnant Human Uterine Layers.bioRxiv [Preprint]. 2023 Dec 20:2023.08.07.551726. doi: 10.1101/2023.08.07.551726. bioRxiv. 2023. Update in: J Mech Behav Biomed Mater. 2024 Mar;151:106348. doi: 10.1016/j.jmbbm.2023.106348. PMID: 37609213 Free PMC article. Updated. Preprint.
References
-
- Ameer MA, Fagan SE, Sosa-Stanley JN, Peterson DC, 2021. Anatomy, abdomen and pelvis, uterus. In: StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK470297/. (Accessed 21 December 2021). - PubMed
-
- Aplin JD, 2003. Implantation. In: Henry HL, Norman AW (Eds.), Encyclopedia of Hormones. Academic Press, pp. 289–297. 10.1016/B0-12-341103-3/00165-0. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

