The glymphatic system and cerebral small vessel disease
- PMID: 38198946
- PMCID: PMC11579894
- DOI: 10.1016/j.jstrokecerebrovasdis.2024.107557
The glymphatic system and cerebral small vessel disease
Abstract
Objectives: Cerebral small vessel disease is a group of pathologies in which alterations of the brain's blood vessels contribute to stroke and neurocognitive changes. Recently, a neurotoxic waste clearance system composed of perivascular spaces abutting the brain's blood vessels, termed the glymphatic system, has been identified as a key player in brain homeostasis. Given that small vessel disease and the glymphatic system share anatomical structures, this review aims to reexamine small vessel disease in the context of the glymphatic system and highlight novel aspects of small vessel disease physiology.
Materials and methods: This review was conducted with an emphasis on studies that examined aspects of small vessel disease and on works characterizing the glymphatic system. We searched PubMed for relevant articles using the following keywords: glymphatics, cerebral small vessel disease, arterial pulsatility, hypertension, blood-brain barrier, endothelial dysfunction, stroke, diabetes.
Results: Cerebral small vessel disease and glymphatic dysfunction are anatomically connected and significant risk factors are shared between the two. These include hypertension, type 2 diabetes, advanced age, poor sleep, obesity, and neuroinflammation. There is clear evidence that CSVD hinders the effective functioning of glymphatic system.
Conclusion: These shared risk factors, as well as the model of cerebral amyloid angiopathy pathogenesis, hint at the possibility that glymphatic dysfunction could independently contribute to the pathogenesis of cerebral small vessel disease. However, the current evidence supports a model of cascading dysfunction, wherein concurrent small vessel and glymphatic injury hinder glymphatic-mediated recovery and promote the progression of subclinical to clinical disease.
Keywords: Cerebrospinal fluid; Dementia; Glymphatic; Perivascular space; Small vessel disease; Stroke.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest James R. Brorson is supported by National Institutes of Health grants R01AG06263 (Aggarwal) and R61NS135583 (Brorson), and has provided medical-legal consultation and consultation to the National Peer Review Corporation.
Figures
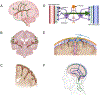
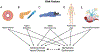

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

