Causes, functions, and therapeutic possibilities of RNA secondary structure ensembles and alternative states
- PMID: 38199037
- PMCID: PMC10842484
- DOI: 10.1016/j.chembiol.2023.12.010
Causes, functions, and therapeutic possibilities of RNA secondary structure ensembles and alternative states
Abstract
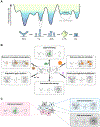
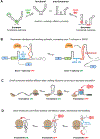
RNA secondary structure plays essential roles in encoding RNA regulatory fate and function. Most RNAs populate ensembles of alternatively paired states and are continually unfolded and refolded by cellular processes. Measuring these structural ensembles and their contributions to cellular function has traditionally posed major challenges, but new methods and conceptual frameworks are beginning to fill this void. In this review, we provide a mechanism- and function-centric compendium of the roles of RNA secondary structural ensembles and minority states in regulating the RNA life cycle, from transcription to degradation. We further explore how dysregulation of RNA structural ensembles contributes to human disease and discuss the potential of drugging alternative RNA states to therapeutically modulate RNA activity. The emerging paradigm of RNA structural ensembles as central to RNA function provides a foundation for a deeper understanding of RNA biology and new therapeutic possibilities.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests A.M.M. is an advisor to and holds equity in RNAConnect and is a consultant to Ribometrix.
Figures




Similar articles
-
The roles of structural dynamics in the cellular functions of RNAs.Nat Rev Mol Cell Biol. 2019 Aug;20(8):474-489. doi: 10.1038/s41580-019-0136-0. Nat Rev Mol Cell Biol. 2019. PMID: 31182864 Free PMC article. Review.
-
Single-molecule fluorescence resonance energy transfer assays reveal heterogeneous folding ensembles in a simple RNA stem-loop.J Mol Biol. 2008 Dec 5;384(1):264-78. doi: 10.1016/j.jmb.2008.08.088. Epub 2008 Sep 13. J Mol Biol. 2008. PMID: 18805425
-
Structural and biophysical dissection of RNA conformational ensembles.Curr Opin Struct Biol. 2024 Oct;88:102908. doi: 10.1016/j.sbi.2024.102908. Epub 2024 Aug 14. Curr Opin Struct Biol. 2024. PMID: 39146886 Review.
-
RNA deadenylation complexes in development and diseases.Biochem Cell Biol. 2023 Apr 1;101(2):131-147. doi: 10.1139/bcb-2022-0325. Epub 2023 Jan 16. Biochem Cell Biol. 2023. PMID: 36645883 Review.
-
Modeling RNA secondary structure folding ensembles using SHAPE mapping data.Nucleic Acids Res. 2018 Jan 9;46(1):314-323. doi: 10.1093/nar/gkx1057. Nucleic Acids Res. 2018. PMID: 29177466 Free PMC article.
Cited by
-
RNA Structure: Past, Future, and Gene Therapy Applications.Int J Mol Sci. 2024 Dec 26;26(1):110. doi: 10.3390/ijms26010110. Int J Mol Sci. 2024. PMID: 39795966 Free PMC article. Review.
-
RNA language models predict mutations that improve RNA function.bioRxiv [Preprint]. 2024 Sep 16:2024.04.05.588317. doi: 10.1101/2024.04.05.588317. bioRxiv. 2024. Update in: Nat Commun. 2024 Dec 5;15(1):10627. doi: 10.1038/s41467-024-54812-y. PMID: 38617247 Free PMC article. Updated. Preprint.
-
SHAPE-based chemical probes for studying preQ1-RNA interactions in living bacteria.bioRxiv [Preprint]. 2025 Jul 21:2025.07.21.665968. doi: 10.1101/2025.07.21.665968. bioRxiv. 2025. PMID: 40777364 Free PMC article. Preprint.
-
5'-UTR G-Quadruplex-Mediated Translation Regulation in Eukaryotes: Current Understanding and Methodological Challenges.Int J Mol Sci. 2025 Jan 30;26(3):1187. doi: 10.3390/ijms26031187. Int J Mol Sci. 2025. PMID: 39940956 Free PMC article. Review.
-
Visualizing RNA structure ensembles by single-molecule correlated chemical probing.Curr Opin Struct Biol. 2024 Oct;88:102877. doi: 10.1016/j.sbi.2024.102877. Epub 2024 Jul 17. Curr Opin Struct Biol. 2024. PMID: 39024941 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

