Increased cellular protein modification by methylglyoxal activates endoplasmic reticulum-based sensors of the unfolded protein response
- PMID: 38199038
- PMCID: PMC10821617
- DOI: 10.1016/j.redox.2024.103025
Increased cellular protein modification by methylglyoxal activates endoplasmic reticulum-based sensors of the unfolded protein response
Abstract
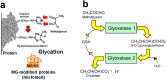
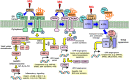
The unfolded protein response (UPR) detects increased misfolded proteins and activates protein refolding, protein degradation and inflammatory responses. UPR sensors in the endoplasmic reticulum, IRE1α and PERK, bind and are activated by proteins with unexpected surface hydrophobicity, whereas sensor ATF6 is activated by proteolytic cleavage when released from complexation with protein disulfide isomerases (PDIs). Metabolic dysfunction leading to the formation of misfolded proteins with surface hydrophobicity and disruption of ATF6-PDI complexes leading to activation of UPR sensors remains unclear. The cellular concentration of reactive dicarbonyl metabolite, methylglyoxal (MG), is increased in impaired metabolic health, producing increased MG-modified cellular proteins. Herein we assessed the effect of high glucose concentration and related increased cellular MG on activation status of IRE1α, PERK and ATF6. Human aortal endothelial cells and HMEC-1 microvascular endothelial cells were incubated in low and high glucose concentration to model blood glucose control, with increase or decrease of MG by silencing or increasing expression of glyoxalase 1 (Glo1), which metabolizes MG. Increased MG induced by high glucose concentration activated IRE1α, PERK and ATF6 and related downstream signalling leading to increased chaperone, apoptotic and inflammatory gene expression. Correction of increased MG by increasing Glo1 expression prevented UPR activation. MG modification of proteins produces surface hydrophobicity through arginine-derived hydroimidazolone MG-H1 formation, with related protein unfolding and preferentially targets PDIs and chaperone pathways for modification. It thereby poses a major challenge to proteostasis and activates UPR sensors. Pharmacological decrease of MG with Glo1 inducer, trans-resveratrol and hesperetin in combination, offers a novel treatment strategy to counter UPR-related cell dysfunction, particularly in hyperglycemia associated with diabetes.
Keywords: ER stress; Glycation; Glycemic disease; Hyperglycemia; Methylglyoxal; Unfolded protein response.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Mingzhan Xue, Naila Rabbani and Paul J Thornalley are co-inventors in the patent of Glo1 inducer, tRES + HESP. Commercial rights to the patent are owned by Glocentrica Ltd (UK). Naila Rabbani and Paul J Thornalley are founding co-directors of Glocentrica Ltd (UK).
Figures




Similar articles
-
Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal.Sci Rep. 2019 May 27;9(1):7889. doi: 10.1038/s41598-019-44358-1. Sci Rep. 2019. PMID: 31133647 Free PMC article.
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
The unfolded protein response is triggered following a single, unaccustomed resistance-exercise bout.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R664-9. doi: 10.1152/ajpregu.00511.2013. Epub 2014 Jul 9. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25009220 Clinical Trial.
-
Dicarbonyl stress, protein glycation and the unfolded protein response.Glycoconj J. 2021 Jun;38(3):331-340. doi: 10.1007/s10719-021-09980-0. Epub 2021 Mar 1. Glycoconj J. 2021. PMID: 33644826 Free PMC article. Review.
-
Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review.
Cited by
-
Methylglyoxal Formation-Metabolic Routes and Consequences.Antioxidants (Basel). 2025 Feb 13;14(2):212. doi: 10.3390/antiox14020212. Antioxidants (Basel). 2025. PMID: 40002398 Free PMC article. Review.
-
Overload of Glucose Metabolism as Initiating Factor in Diabetic Embryopathy and Prevention by Glyoxalase 1 Inducer Dietary Supplement.Antioxidants (Basel). 2025 Aug 21;14(8):1022. doi: 10.3390/antiox14081022. Antioxidants (Basel). 2025. PMID: 40867918 Free PMC article.
-
Glyoxalase 1 Inducer, trans-Resveratrol and Hesperetin-Dietary Supplement with Multi-Modal Health Benefits.Antioxidants (Basel). 2025 Aug 4;14(8):956. doi: 10.3390/antiox14080956. Antioxidants (Basel). 2025. PMID: 40867852 Free PMC article.
-
Exploring Glyoxalase Strategies for Managing Sugar-Induced Chronic Diseases.Life (Basel). 2025 May 16;15(5):794. doi: 10.3390/life15050794. Life (Basel). 2025. PMID: 40430220 Free PMC article.
-
Polysulfide and persulfide-mediated activation of the PERK-eIF2α-ATF4 pathway increases Sestrin2 expression and reduces methylglyoxal toxicity.Redox Biol. 2025 Feb;79:103450. doi: 10.1016/j.redox.2024.103450. Epub 2024 Dec 5. Redox Biol. 2025. PMID: 39667306 Free PMC article.
References
-
- Fatima S., Ambreen S., Mathew A., Elwakiel A., Gupta A., Singh K., Krishnan S., Rana R., Khawaja H., Gupta D., Manoharan J., Besler C., Laufs U., Kohli S., Isermann B., Shahzad K. ER-Stress and senescence coordinately promote endothelial barrier dysfunction in diabetes-induced atherosclerosis. Nutrients. 2022;14:2786. doi: 10.3390/nu14142786. - DOI - PMC - PubMed
-
- Quagliaro L., Piconi L., Assaloni R., Da Ros R., Maier A., Zuodar G., Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005;183:259–267. doi: 10.1016/j.atherosclerosis.2005.03.015. - DOI - PubMed
-
- Xue M., Qian Q., Adaikalakoteswari A., Rabbani N., Babaei-Jadidi R., Thornalley P.J. Activation of NF-E2-Related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. 0.2337/db06-1003. Epub 2008 Jul 15. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

