Longevity control by supersulfide-mediated mitochondrial respiration and regulation of protein quality
- PMID: 38199039
- PMCID: PMC10821618
- DOI: 10.1016/j.redox.2023.103018
Longevity control by supersulfide-mediated mitochondrial respiration and regulation of protein quality
Abstract
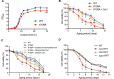
Supersulfides, which are defined as sulfur species with catenated sulfur atoms, are increasingly being investigated in biology. We recently identified pyridoxal phosphate (PLP)-dependent biosynthesis of cysteine persulfide (CysSSH) and related supersulfides by cysteinyl-tRNA synthetase (CARS). Here, we investigated the physiological role of CysSSH in budding yeast (Saccharomyces cerevisiae) by generating a PLP-binding site mutation K109A in CRS1 (the yeast ortholog of CARS), which decreased the synthesis of CysSSH and related supersulfides and also led to reduced chronological aging, effects that were associated with an increased endoplasmic reticulum stress response and impaired mitochondrial bioenergetics. Reduced chronological aging in the K109A mutant could be rescued by using exogenous supersulfide donors. Our findings indicate important roles for CARS in the production and metabolism of supersulfides-to mediate mitochondrial function and to regulate longevity.
Keywords: Cysteinyl-tRNA synthetase; ER stress; Longevity; Mitochondrial energy metabolism; Supersulfides.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures





References
-
- Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N.O., Xian M., Fukuto J.M., Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111(21):7606–7611. - PMC - PubMed
-
- Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J.M., Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8(1):1177. - PMC - PubMed
-
- Ono K., Akaike T., Sawa T., Kumagai Y., Wink D.A., Tantillo D.J., Hobbs A.J., Nagy P., Xian M., Lin J., Fukuto J.M. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014;77:82–94. - PMC - PubMed
-
- Jung M., Kasamatsu S., Matsunaga T., Akashi S., Ono K., Nishimura A., Morita M., Abdul Hamid H., Fujii S., Kitamura H., Sawa T., Ida T., Motohashi H., Akaike T. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016;480(2):180–186. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

