Design, synthesis, and evaluation of dual EGFR/AURKB inhibitors as anticancer agents for non-small cell lung cancer
- PMID: 38199330
- PMCID: PMC10951975
- DOI: 10.1016/j.bmcl.2024.129612
Design, synthesis, and evaluation of dual EGFR/AURKB inhibitors as anticancer agents for non-small cell lung cancer
Abstract
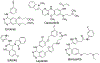
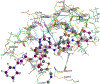
The epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are first-line agents for mutant EGFR-positive (mEGFR+) NSCLC. However, secondary resistant mutations develop following therapy that prevent EGFR-TKI binding. The EGFR-TKIs are rendered ineffective in NSCLC expressing EGFR resistant mutations (rmEGFR+). Mutations in Kirsten rat sarcoma virus protein (mKRAS) support persistent signaling downstream of EGFR regardless of EGFR-TKI earlier in the signaling cascade. The EGFR-TKIs are ineffective in mKRAS+ NSCLC. Thus, newer anticancer agents are needed for rmEGFR+ and mKRAS+ NSCLC. Aurora kinase B (AURKB) is a mitosis related kinase that is overexpressed in NSCLC and supports cancer cell proliferation and survival. Literature reports have suggested that AURKB inhibitors if given concurrently with an EGFR-TKI could overcome EGFR-TKI resistance in mKRAS+ NSCLC and rmEGFR + NSCLC, and showed improved anticancer effects compared to current single-targeted EGFR-TKIs. Molecular modeling was used to identify similarities between the kinase pockets of EGFR and AURKB. An overlap was observed for the inactive conformation of EGFR and the active conformation of AURKB. Compounds 3-7 were synthesized as dual EGFR/AURKB inhibitors for mKRAS+ and rmEGFR+ NSCLC. Compounds 5, 6 and 7 were identified as dual EGFR/AURKB inhibitors. Compound 5 demonstrated modest micromolar inhibition of rmEGFR+ NSCLC. All investigated compounds showed moderate inhibition of mKRAS+ NSCLC cells. Compound 7 demonstrated single-digit micromolar inhibition of mKRAS+ NSCLC.
Keywords: Aurora kinase; Epidermal growth factor receptor kinase; Lung cancer; Resistance.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


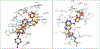

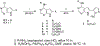
References
-
- Yarden Y, Sliwkowski M. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127. - PubMed
-
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958. - PubMed
-
- http://www.cancer.org/cancer/lungcancer/ Last accessed: Dec 16, 2023.
-
- Downward J Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

