HDAC6/aggresome processing pathway importance for inflammasome formation is context-dependent
- PMID: 38199570
- PMCID: PMC10850954
- DOI: 10.1016/j.jbc.2024.105638
HDAC6/aggresome processing pathway importance for inflammasome formation is context-dependent
Abstract
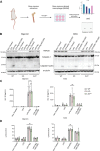
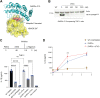
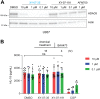
The inflammasome is a large multiprotein complex that assembles in the cell cytoplasm in response to stress or pathogenic infection. Its primary function is to defend the cell and promote the secretion of pro-inflammatory cytokines, including IL-1β and IL-18. Previous research has shown that in immortalized bone marrow-derived macrophages (iBMDMs) inflammasome assembly is dependent on the deacetylase HDAC6 and the aggresome processing pathway (APP), a cellular pathway involved in the disposal of misfolded proteins. Here we used primary BMDMs from mice in which HDAC6 is ablated or impaired and found that inflammasome activation was largely normal. We also used human peripheral blood mononuclear cells and monocyte cell lines expressing a synthetic protein blocking the HDAC6-ubiquitin interaction and impairing the APP and found that inflammasome activation was moderately affected. Finally, we used a novel HDAC6 degrader and showed that inflammasome activation was partially impaired in human macrophage cell lines with depleted HDAC6. Our results therefore show that HDAC6 importance in inflammasome activation is context-dependent.
Keywords: DARPin; HDAC6; degrader; inflammasome; inhibitors; interleukin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Part of the results presented herein have been used in the patent application EP-A-20213494.6. A. U., S. A., C. J. F. and F. B. are employees of Novartis. E. S. F is a founder, scientific advisory board (SAB) member, and equity holder of Civetta Therapeutics, Lighthorse Therapeutics, Proximity Therapeutics, and Neomorph, Inc. (also board of directors). He is an equity holder and SAB member for Avilar Therapeutics and Photys Therapeutics and a consultant to Novartis, Sanofi, EcoR1 Capital, Ajax Therapeutics, Odyssey Therapeutics and Deerfield. The Fischer lab receives or has received research funding from Deerfield, Novartis, Ajax, Interline and Astellas. K. A. D is a consultant to Kronos Bio and Neomorph Inc.
Figures

Update of
-
HDAC6/aggresome processing pathway importance for inflammasome formation is context dependent.bioRxiv [Preprint]. 2023 Aug 15:2023.08.15.553363. doi: 10.1101/2023.08.15.553363. bioRxiv. 2023. Update in: J Biol Chem. 2024 Feb;300(2):105638. doi: 10.1016/j.jbc.2024.105638. PMID: 37645730 Free PMC article. Updated. Preprint.
References
-
- Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P., et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. - PubMed
-
- Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16:407–420. - PubMed
-
- Perregaux D., Gabel C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. - PubMed
-
- Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

