Chromatin targeting of the RNF12/RLIM E3 ubiquitin ligase controls transcriptional responses
- PMID: 38199845
- PMCID: PMC10781586
- DOI: 10.26508/lsa.202302282
Chromatin targeting of the RNF12/RLIM E3 ubiquitin ligase controls transcriptional responses
Abstract
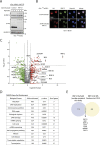
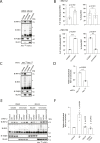
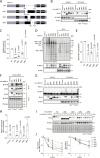
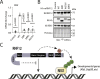
Protein ubiquitylation regulates key biological processes including transcription. This is exemplified by the E3 ubiquitin ligase RNF12/RLIM, which controls developmental gene expression by ubiquitylating the REX1 transcription factor and is mutated in an X-linked intellectual disability disorder. However, the precise mechanisms by which ubiquitylation drives specific transcriptional responses are not known. Here, we show that RNF12 is recruited to specific genomic locations via a consensus sequence motif, which enables co-localisation with REX1 substrate at gene promoters. Surprisingly, RNF12 chromatin recruitment is achieved via a non-catalytic basic region and comprises a previously unappreciated N-terminal autoinhibitory mechanism. Furthermore, RNF12 chromatin targeting is critical for REX1 ubiquitylation and downstream RNF12-dependent gene regulation. Our results demonstrate a key role for chromatin in regulation of the RNF12-REX1 axis and provide insight into mechanisms by which protein ubiquitylation enables programming of gene expression.
© 2024 Espejo-Serrano et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
