Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage
- PMID: 38200313
- PMCID: PMC10808075
- DOI: 10.1038/s41586-023-06899-4
Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage
Abstract
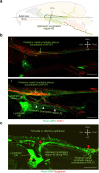
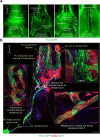
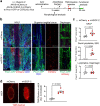
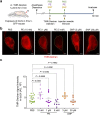
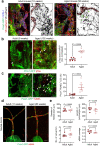
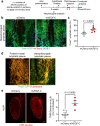
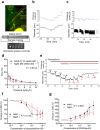
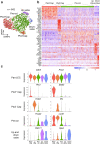
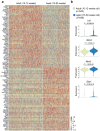
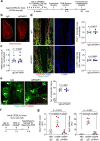
Cerebrospinal fluid (CSF) in the subarachnoid space around the brain has long been known to drain through the lymphatics to cervical lymph nodes1-17, but the connections and regulation have been challenging to identify. Here, using fluorescent CSF tracers in Prox1-GFP lymphatic reporter mice18, we found that the nasopharyngeal lymphatic plexus is a major hub for CSF outflow to deep cervical lymph nodes. This plexus had unusual valves and short lymphangions but no smooth-muscle coverage, whereas downstream deep cervical lymphatics had typical semilunar valves, long lymphangions and smooth muscle coverage that transported CSF to the deep cervical lymph nodes. α-Adrenergic and nitric oxide signalling in the smooth muscle cells regulated CSF drainage through the transport properties of deep cervical lymphatics. During ageing, the nasopharyngeal lymphatic plexus atrophied, but deep cervical lymphatics were not similarly altered, and CSF outflow could still be increased by adrenergic or nitric oxide signalling. Single-cell analysis of gene expression in lymphatic endothelial cells of the nasopharyngeal plexus of aged mice revealed increased type I interferon signalling and other inflammatory cytokines. The importance of evidence for the nasopharyngeal lymphatic plexus functioning as a CSF outflow hub is highlighted by its regression during ageing. Yet, the ageing-resistant pharmacological activation of deep cervical lymphatic transport towards lymph nodes can still increase CSF outflow, offering an approach for augmenting CSF clearance in age-related neurological conditions in which greater efflux would be beneficial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

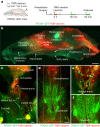




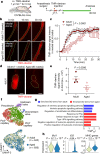

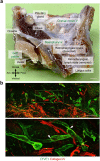
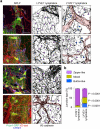


Comment in
-
A nasal hub for cerebrospinal fluid clearance.Nat Cardiovasc Res. 2024 Feb;3(2):98-99. doi: 10.1038/s44161-024-00423-1. Nat Cardiovasc Res. 2024. PMID: 39196192 No abstract available.
References
-
- Key, A. & Retzius, G. Studien in der Anatomie des Nervensystems und des Bindegewebes: 1. Hälfte Vol. 1 (Samson & Wallin, 1875).
-
- Bradbury M, Cserr H. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. Exp. Biol. Lymph. Circ. 1985;9:355–394.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

