Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: results from the FIREFLEYE randomized phase 3 study
- PMID: 38200320
- PMCID: PMC11126565
- DOI: 10.1038/s41433-023-02919-9
Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: results from the FIREFLEYE randomized phase 3 study
Erratum in
-
Correction: Systemic exposure to aflibercept after intravitreal injection in premature neonates with retinopathy of prematurity: results from the FIREFLEYE randomized phase 3 study.Eye (Lond). 2024 Jun;38(8):1599-1600. doi: 10.1038/s41433-024-02948-y. Eye (Lond). 2024. PMID: 38332376 Free PMC article. No abstract available.
Abstract
Background: There are no data on pharmacokinetics, pharmacodynamics, and immunogenicity of intravitreal aflibercept in preterm infants with retinopathy of prematurity (ROP). FIREFLEYE compared aflibercept 0.4 mg/eye and laser photocoagulation in infants with acute-phase ROP requiring treatment.
Methods: Infants (gestational age ≤32 weeks or birthweight ≤1500 g) with treatment-requiring ROP in ≥1 eye were randomized 2:1 to receive aflibercept 0.4 mg or laser photocoagulation at baseline in this 24-week, randomized, open-label, noninferiority, phase 3 study. Endpoints include concentrations of free and adjusted bound aflibercept in plasma, pharmacokinetic/pharmacodynamic exploration of systemic anti-vascular endothelial growth factor effects, and immunogenicity.
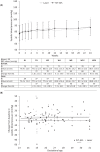
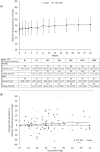
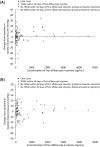
Results: Of 113 treated infants, 75 received aflibercept 0.4 mg per eye at baseline (mean chronological age: 10.4 weeks), mostly bilaterally (71 infants), and with 1 injection/eye (120/146 eyes). Concentrations of free aflibercept were highly variable, with maximum concentration at day 1, declining thereafter. Plasma concentrations of adjusted bound (pharmacologically inactive) aflibercept increased from day 1 to week 4, decreasing up to week 24. Six infants experienced treatment-emergent serious adverse events within 30 days of treatment; aflibercept concentrations were within the range observed in other infants. There was no pattern between free and adjusted bound aflibercept concentrations and blood pressure changes up to week 4. A low-titer (1:30), non-neutralizing, treatment-emergent anti-drug antibody response was reported in 1 infant, though was not clinically relevant.
Conclusions: 24-week data suggest intravitreal aflibercept for treatment of acute-phase ROP is not associated with clinically relevant effects on blood pressure, further systemic adverse events, or immunogenicity.
Gov identifier: NCT04004208.
© 2024. The Author(s).
Conflict of interest statement
AS: Alcon (scientific advisory boards), Allergan (speaker), Apellis (scientific advisory boards), Bayer (speaker, participation in clinical trials, research grants and scientific advisory boards), Novartis (speaker, participation in clinical trials, research grants and scientific advisory boards), Roche (speaker, scientific advisory boards), SemaThera (Board of Directors). NA: Bayer (participation in clinical trials) and Novartis (participation in clinical trials). W-CW: Bayer (participation in clinical trials) and Novartis (participation in clinical trials) DL: Bayer (participation in clinical trials) and Novartis (participation in clinical trials). ES: Allergan (Speaker), Bayer (participation in clinical trials), Novartis (participation in clinical trials), and TR-PHARM (participation in clinical trials) HN: Nothing to disclose JM: AbbVie (speaker), AstraZeneca (speaker), Bayer (participation in clinical trials), Draeger (speaker), HIPP (speaker), Maquet (speaker), Merck Sharp & Dohme (participation in clinical trials), Nestle (speaker), Nutricia (speaker), Roche (speaker), and WindTree (participation in clinical trials) SL: Employee of Bayer Consumer Care AG, Basel, Switzerland AP: Employee of Chrestos Concept GmbH & Co, Essen, Germany. SS: Employee of Bayer AG, Berlin, Germany. TE: Employee and shareholder of Bayer AG, Leverkusen, Germany. KT: Employee of Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA. AZ: Employee of Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA. JW: Employee of OCCAMS, Amstelveen, The Netherlands. JH: Employee of Bayer AG, Berlin, Germany. EK: Employee of Bayer AG, Berlin, Germany. TZ: Former employee of Bayer AG, Berlin, Germany; retired August 2023.
Figures

References
-
- Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association For Pediatric Ophthalmology and Strabismus, American Association Of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142:e20183061. doi: 10.1542/peds.2018-3061. - DOI - PubMed
-
- Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am J Pathol. 2000;156:1337–44. doi: 10.1016/S0002-9440(10)65004-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

