NON-pharmacological Approach Less Invasive Surfactant Administration (NONA-LISA) trial: protocol for a randomised controlled trial
- PMID: 38200325
- PMCID: PMC11502479
- DOI: 10.1038/s41390-023-02998-0
NON-pharmacological Approach Less Invasive Surfactant Administration (NONA-LISA) trial: protocol for a randomised controlled trial
Abstract
Introduction: Using pre-procedure analgesia with the risk of apnoea may complicate the Less Invasive Surfactant Administration (LISA) procedure or reduce the effect of LISA.
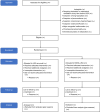
Methods: The NONA-LISA trial (ClinicalTrials.gov, NCT05609877) is a multicentre, blinded, randomised controlled trial aiming at including 324 infants born before 30 gestational weeks, meeting the criteria for surfactant treatment by LISA. Infants will be randomised to LISA after administration of fentanyl 0.5-1 mcg/kg intravenously (fentanyl group) or isotonic saline solution intravenously (saline group). All infants will receive standardised non-pharmacological comfort care before and during the LISA procedure. Additional analgesics will be provided at the clinician's discretion. The primary outcome is the need for invasive ventilation, meaning mechanical or manual ventilation via an endotracheal tube, for at least 30 min (cumulated) within 24 h of the procedure. Secondary outcomes include the modified COMFORTneo score during the procedure, bronchopulmonary dysplasia at 36 weeks, and mortality at 36 weeks.
Discussion: The NONA-LISA trial has the potential to provide evidence for a standardised approach to relief from discomfort in preterm infants during LISA and to reduce invasive ventilation. The results may affect future clinical practice.
Impact: Pre-procedure analgesia is associated with apnoea and may complicate procedures that rely on regular spontaneous breathing, such as Less Invasive Surfactant Administration (LISA). This randomised controlled trial addresses the effect of analgesic premedication in LISA by comparing fentanyl with a placebo (isotonic saline) in infants undergoing the LISA procedure. All infants will receive standardised non-pharmacological comfort. The NONA-LISA trial has the potential to provide evidence for a standardised approach to relief from discomfort or pain in preterm infants during LISA and to reduce invasive ventilation. The results may affect future clinical practice regarding analgesic treatment associated with the LISA procedure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Kribs, A. Minimally invasive surfactant therapy and noninvasive respiratory support. Clin. Perinatol.43, 755–771 (2016). - PubMed
-
- Polin, R. A. & Carlo, W. A., COMMITTEE ON FETUS AND NEWBORN. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics133, 156–163 (2014). - PubMed
-
- Herting, E. Less Invasive Surfactant Administration (LISA) — ways to deliver surfactant in spontaneously breathing infants. Early Hum. Dev.89, 875–880 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical