Tobacco and menthol flavored nicotine-free electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium
- PMID: 38200492
- PMCID: PMC10777495
- DOI: 10.1186/s12931-023-02537-9
Tobacco and menthol flavored nicotine-free electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium
Abstract
Background: Electronic cigarette (e-cig) vaping has increased in the past decade in the US, and e-cig use is misleadingly marketed as a safe cessation for quitting smoking. The main constituents in e-liquid are humectants, such as propylene glycol (PG) and vegetable glycerine (VG), but different flavoring chemicals are also used. However, the toxicology profile of flavored e-cigs in the pulmonary tract is lacking. We hypothesized that menthol and tobacco-flavored e-cig (nicotine-free) exposure results in inflammatory responses and dysregulated repair in lung fibroblast and epithelium.
Method: We exposed lung fibroblast (HFL-1) and epithelium (BEAS-2B) to Air, PG/VG, menthol flavored, or tobacco-flavored e-cig, and determined the cytotoxicity, inflammation, and wound healing ability in 2D cells and 3D microtissue chip models.
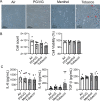
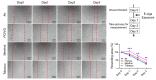
Results: After exposure, HFL-1 showed decreased cell number with increased IL-8 levels in the tobacco flavor group compared to air. BEAS-2B also showed increased IL-8 secretion after PG/VG and tobacco flavor exposure, while menthol flavor exposure showed no change. Both menthol and tobacco-flavored e-cig exposure showed decreased protein abundance of type 1 collagen α 1 (COL1A1), α-smooth-muscle actin (αSMA), and fibronectin as well as decreased gene expression level of αSMA (Acta2) in HFL-1. After tobacco flavor e-cig exposure, HFL-1 mediated wound healing and tissue contractility were inhibited. Furthermore, BEAS-2B exposed to menthol flavor showed significantly decreased tight junction gene expressions, such as CDH1, OCLN, and TJP1.
Conclusion: Overall, tobacco-flavored e-cig exposure induces inflammation in both epithelium and fibroblasts, and tobacco-flavored e-cig inhibits wound healing ability in fibroblasts.
Keywords: ENDS; Inflammation; Injury; Menthol; Nicotine-free; Repair; Tobacco.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Figures




Update of
-
Tobacco and Menthol flavored electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium.Res Sq [Preprint]. 2023 Jun 12:rs.3.rs-3037297. doi: 10.21203/rs.3.rs-3037297/v1. Res Sq. 2023. Update in: Respir Res. 2024 Jan 10;25(1):23. doi: 10.1186/s12931-023-02537-9. PMID: 37398084 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

