ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
- PMID: 38200543
- PMCID: PMC10777643
- DOI: 10.1186/s12964-023-01399-4
ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease
Abstract
Background: Cardiolipin (CL) plays a critical role in maintaining mitochondrial membrane integrity and overall mitochondrial homeostasis. Recent studies have suggested that mitochondrial damage resulting from abnormal cardiolipin remodelling is associated with the pathogenesis of diabetic kidney disease (DKD). Acyl-coenzyme A:lyso-cardiolipin acyltransferase-1 (ALCAT1) was confirmed to be involved in the progression of Parkinson's disease, diet-induced obesity and other ageing-related diseases by regulating pathological cardiolipin remodelling. Thus, the purpose of this investigation was to determine the role of ALCAT1-mediated CL remodelling in DKD and to explore the potential underlying mechanism.
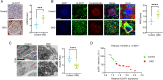
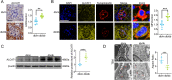
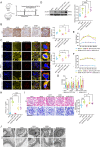
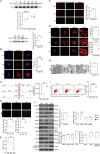
Methods: In vivo study, the mitochondrial structure was examined by transmission electron microscopy (TEM). The colocalization of ALCAT1 and synaptopodin was evaluated by double immunolabelling. Western blotting (WB) was performed to assess ALCAT1 expression in glomeruli. Lipidomics analysis was conducted to evaluate the composition of reconstructed cardiolipins. In vitro study, the lipidomics, TEM and WB analyses were similar to those in vivo. Mitochondrial function was evaluated by measuring the mitochondrial membrane potential (MMP) and the production of ATP and ROS.
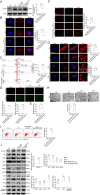
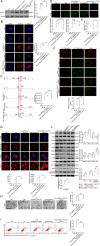
Results: Here, we showed that increased oxidized cardiolipin (ox-CL) and significant mitochondrial damage were accompanied by increased ALCAT1 expression in the glomeruli of patients with DKD. Similar results were found in db/db mouse kidneys and in cultured podocytes stimulated with high glucose (HG). ALCAT1 deficiency effectively prevented HG-induced ox-CL production and mitochondrial damage in podocytes. In contrast, ALCAT1 upregulation enhanced ox-CL levels and podocyte mitochondrial dysfunction. Moreover, treatment with the cardiolipin antioxidant SS-31 markedly inhibited mitochondrial dysfunction and cell injury, and SS-31 treatment partly reversed the damage mediated by ALCAT1 overexpression. We further found that ALCAT1 could mediate the key regulators of mitochondrial dynamics and mitophagy through the AMPK pathway.
Conclusions: Collectively, our studies demonstrated that ALCAT1-mediated cardiolipin remodelling played a crucial role in DKD, which might provide new insights for DKD treatment. Video Abstract.
Keywords: ALCAT1; Cardiolipin remodelling; Diabetic kidney disease; Mitochondrial dysfunction; Oxidized cardiolipin; Podocyte injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes.J Clin Invest. 2019 Jul 22;129(8):3387-3400. doi: 10.1172/JCI125316. eCollection 2019 Jul 22. J Clin Invest. 2019. PMID: 31329164 Free PMC article.
-
Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson's diseases.Aging Cell. 2019 Jun;18(3):e12941. doi: 10.1111/acel.12941. Epub 2019 Mar 5. Aging Cell. 2019. PMID: 30838774 Free PMC article.
-
Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity.Cell Metab. 2010 Aug 4;12(2):154-65. doi: 10.1016/j.cmet.2010.07.003. Cell Metab. 2010. PMID: 20674860 Free PMC article.
-
In Search of the Holy Grail: Toward a Unified Hypothesis on Mitochondrial Dysfunction in Age-Related Diseases.Cells. 2022 Jun 12;11(12):1906. doi: 10.3390/cells11121906. Cells. 2022. PMID: 35741033 Free PMC article. Review.
-
Cardiolipin Remodeling in Cardiovascular Diseases: Implication for Mitochondrial Dysfunction.Acta Physiol (Oxf). 2025 Jul;241(7):e70073. doi: 10.1111/apha.70073. Acta Physiol (Oxf). 2025. PMID: 40530586 Review.
Cited by
-
Analysis of neuronal cardiolipin and monolysocardiolipin from biological samples with cyclic ion mobility mass spectrometry.Front Physiol. 2025 May 29;16:1592008. doi: 10.3389/fphys.2025.1592008. eCollection 2025. Front Physiol. 2025. PMID: 40510267 Free PMC article.
-
Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) Ameliorate Heart Failure through Reductions in Oxidative Stress: A Systematic Review and Meta-Analysis.Antioxidants (Basel). 2024 Aug 6;13(8):955. doi: 10.3390/antiox13080955. Antioxidants (Basel). 2024. PMID: 39199201 Free PMC article. Review.
-
The role of natural products in improving lipid metabolism disorder-induced mitochondrial dysfunction of diabetic kidney disease.Front Physiol. 2025 Jun 24;16:1624077. doi: 10.3389/fphys.2025.1624077. eCollection 2025. Front Physiol. 2025. PMID: 40630391 Free PMC article. Review.
-
Roles of Mitochondrial Dysfunction in Diabetic Kidney Disease: New Perspectives from Mechanism to Therapy.Biomolecules. 2024 Jun 20;14(6):733. doi: 10.3390/biom14060733. Biomolecules. 2024. PMID: 38927136 Free PMC article. Review.
-
The intelligent podocyte: sensing and responding to a complex microenvironment.Nat Rev Nephrol. 2025 Jul;21(7):503-516. doi: 10.1038/s41581-025-00965-y. Epub 2025 May 8. Nat Rev Nephrol. 2025. PMID: 40341763 Review.
References
-
- Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, et al. Trends in chronic Kidney Disease in China. N Engl J Med. 2016;375:905–6. - PubMed
-
- Ma RCW. Epidemiology of Diabetes and diabetic Complications in China. Diabetologia. 2018;61:1249–60. - PubMed
-
- DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic Kidney Disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319–34. - PubMed
-
- Guedes M, Pecoits-Filho R. Can we cure diabetic Kidney Disease? Present and future perspectives from a nephrologist’s point of view. J Intern Med. 2022;291:165–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

