Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma
- PMID: 38200580
- PMCID: PMC10782703
- DOI: 10.1186/s13046-024-02944-w
Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma
Abstract
Background: Medulloblastoma (MB) patients with MYC oncogene amplification or overexpression exhibit extremely poor prognoses and therapy resistance. However, MYC itself has been one of the most challenging targets for cancer treatment. Here, we identify a novel marinopyrrole natural derivative, MP1, that shows desirable anti-MYC and anti-cancer activities in MB.
Methods: In this study, using MYC-amplified (Group 3) and non-MYC amplified MB cell lines in vitro and in vivo, we evaluated anti-cancer efficacies and molecular mechanism(s) of MP1.
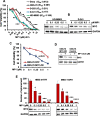
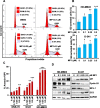
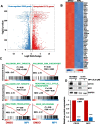
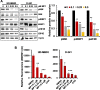
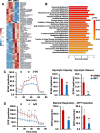
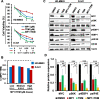
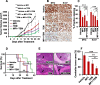
Results: MP1 significantly suppressed MB cell growth and sphere counts and induced G2 cell cycle arrest and apoptosis in a MYC-dependent manner. Mechanistically, MP1 strongly downregulated the expression of MYC protein. Our results with RNA-seq revealed that MP1 significantly modulated global gene expression and inhibited MYC-associated transcriptional targets including translation/mTOR targets. In addition, MP1 inhibited MYC-target metabolism, leading to declined energy levels. The combination of MP1 with an FDA-approved mTOR inhibitor temsirolimus synergistically inhibited MB cell growth/survival by downregulating the expression of MYC and mTOR signaling components. Our results further showed that as single agents, both MP1 and temsirolimus, were able to significantly inhibit tumor growth and MYC expression in subcutaneously or orthotopically MYC-amplified MB bearing mice. In combination, there were further anti-MB effects on the tumor growth and MYC expression in mice.
Conclusion: These preclinical findings highlight the promise of marinopyrrole MP1 as a novel MYC inhibition approach for MYC-amplified MB.
Keywords: MP1; MYC; Marinopyrroles; Medulloblastoma; Metabolism; mTOR/translation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

