Renal Mitochondrial ATP Transporter Ablation Ameliorates Obesity-Induced CKD
- PMID: 38200648
- PMCID: PMC10914206
- DOI: 10.1681/ASN.0000000000000294
Renal Mitochondrial ATP Transporter Ablation Ameliorates Obesity-Induced CKD
Abstract
Significance statement: This study sheds light on the central role of adenine nucleotide translocase 2 (ANT2) in the pathogenesis of obesity-induced CKD. Our data demonstrate that ANT2 depletion in renal proximal tubule cells (RPTCs) leads to a shift in their primary metabolic program from fatty acid oxidation to aerobic glycolysis, resulting in mitochondrial protection, cellular survival, and preservation of renal function. These findings provide new insights into the underlying mechanisms of obesity-induced CKD and have the potential to be translated toward the development of targeted therapeutic strategies for this debilitating condition.
Background: The impairment in ATP production and transport in RPTCs has been linked to the pathogenesis of obesity-induced CKD. This condition is characterized by kidney dysfunction, inflammation, lipotoxicity, and fibrosis. In this study, we investigated the role of ANT2, which serves as the primary regulator of cellular ATP content in RPTCs, in the development of obesity-induced CKD.
Methods: We generated RPTC-specific ANT2 knockout ( RPTC-ANT2-/- ) mice, which were then subjected to a 24-week high-fat diet-feeding regimen. We conducted comprehensive assessment of renal morphology, function, and metabolic alterations of these mice. In addition, we used large-scale transcriptomics, proteomics, and metabolomics analyses to gain insights into the role of ANT2 in regulating mitochondrial function, RPTC physiology, and overall renal health.
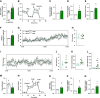
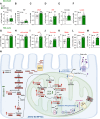
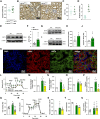
Results: Our findings revealed that obese RPTC-ANT2-/- mice displayed preserved renal morphology and function, along with a notable absence of kidney lipotoxicity and fibrosis. The depletion of Ant2 in RPTCs led to a fundamental rewiring of their primary metabolic program. Specifically, these cells shifted from oxidizing fatty acids as their primary energy source to favoring aerobic glycolysis, a phenomenon mediated by the testis-selective Ant4.
Conclusions: We propose a significant role for RPTC-Ant2 in the development of obesity-induced CKD. The nullification of RPTC-Ant2 triggers a cascade of cellular mechanisms, including mitochondrial protection, enhanced RPTC survival, and ultimately the preservation of kidney function. These findings shed new light on the complex metabolic pathways contributing to CKD development and suggest potential therapeutic targets for this condition.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
H. Amal reports Consultancy: Beyond Air. Inc. and Point6 Bio Ltd.; Ownership Interest: Beyond Air. Inc.; Research Funding: Beyond Air. Inc.; and Patents or Royalties: Hebrew University. M. Berger reports Patents or Royalties: Yissum. R. Cinar reports Patents or Royalties co-inventor for US patents as being US Federal Government employee. G. Kunos reports Patents or Royalties: Inversago Pharma, Inc. G. Leibowitz reports Consultancy: AstraZeneca, Medtronic, Merck-Serono, and Novo Nordik. J. Tam reports Consultancy: BioNanoSim and SciSpark; Ownership Interest: BioNanoSim, Clearmind Medicine, and EPM; Research Funding: BioNanoSim, Clearmind Medicine, and EPM; and Advisory or Leadership Role: BioNanoSim, EPM, and SciSpark. All remaining authors have nothing to disclose.
Figures





Comment in
-
Adenine Nucleotide Translocators Control Kidney Metabolism and Lipid Handling.J Am Soc Nephrol. 2024 Mar 1;35(3):257-258. doi: 10.1681/ASN.0000000000000314. J Am Soc Nephrol. 2024. PMID: 38356156 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

