Outcomes of SARS-CoV-2 infection in early pregnancy-A systematic review and meta-analysis
- PMID: 38200686
- PMCID: PMC11019531
- DOI: 10.1111/aogs.14764
Outcomes of SARS-CoV-2 infection in early pregnancy-A systematic review and meta-analysis
Abstract
Introduction: Available data on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and pregnancy outcomes mostly refer to women contracting the infection during advanced pregnancy or close to delivery. There is limited information on the association between SARS-CoV-2 infection in early pregnancy and outcomes thereof.
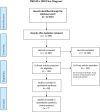
Material and methods: We aimed to systematically review the maternal, fetal and neonatal outcomes following SARS-CoV-2 infection in early pregnancy, defined as <20 weeks of gestation (PROSPERO Registration 2020 CRD42020177673). Searches were carried out in PubMed, Medline, EMBASE, and Scopus databases from January 2020 until April 2023 and the WHO database of publications on coronavirus disease 2019 (COVID-19) from December 2019 to April 2023. Cohort and case-control studies on COVID-19 occurring in early pregnancy that reported data on maternal, fetal, and neonatal outcomes were included. Case reports and studies reporting only exposure to SARS-CoV-2 or not stratifying outcomes based on gestational age were excluded. Data were extracted in duplicate. Meta-analyses were conducted when appropriate, using R meta (R version 4.0.5).
Results: A total of 18 studies, 12 retrospective and six prospective, were included in this review, reporting on 10 147 SARS-CoV-2-positive women infected in early pregnancy, 9533 neonates, and 180 882 SARS-CoV-2 negative women. The studies had low to moderate risk of bias according to the Newcastle-Ottawa quality assessment Scale. The studies showed significant clinical and methodological heterogeneity. A meta-analysis could be performed only on the outcome miscarriage rate, with a pooled random effect odds ratio of 1.44 (95% confidence interval 0.96-2.18), showing no statistical difference in miscarriage in SARS-CoV-2-infected women. Individual studies reported increased incidences of stillbirth, low birthweight and preterm birth among neonates born to mothers affected by COVID-19 in early pregnancy; however, these results were not consistent among all studies.
Conclusions: In this comprehensive systematic review of available evidence, we identified no statistically significant adverse association between SARS-CoV-2 infection in early pregnancy (before 20 weeks of gestation) and fetal, neonatal, or maternal outcomes. However, a 44% increase in miscarriage rate is concerning and further studies of larger sample size are needed to confirm or refute our findings.
Keywords: COVID‐19; early complications; miscarriage; pregnancy; stillbirth.
© 2024 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
The authors have no conflicts of interest to declare in connection with this article.
Figures

References
-
- WAPM (World Association of Perinatal Medicine) Working Group on COVID‐19 . Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection. Ultrasound Obstet Gynecol. 2021;57(2):232‐241. Erratum in: Ultrasound Obstet Gynecol. 202;58(3):496. - PubMed
-
- Mullins E, Hudak ML, Banerjee J, et al. PAN‐COVID investigators and the National Perinatal COVID‐19 registry study group. Pregnancy and neonatal outcomes of COVID‐19: coreporting of common outcomes from PAN‐COVID and AAP‐SONPM registries. Ultrasound Obstet Gynecol. 2021;57(4):573‐581. - PMC - PubMed
-
- Strid P, Zapata LB, Tong VT, et al. Coronavirus disease 2019 (COVID‐19) severity among women of reproductive age with symptomatic laboratory‐confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection by pregnancy status‐United States, 1 January 2020‐25 December 2021. Clin Infect Dis. 2022;75(Suppl 2):S317‐S325. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

