Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation
- PMID: 38200818
- PMCID: PMC10778526
- DOI: 10.3390/ani14010087
Post-Thaw Storage Temperature Influenced Boar Sperm Quality and Lifespan through Apoptosis and Lipid Peroxidation
Abstract
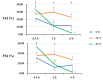
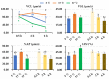
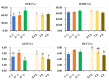
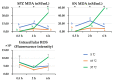
Cryopreservation deteriorates boar sperm quality and lifespan, which restricts the use of artificial insemination with frozen-thawed boar semen in field conditions. The objective of this study was to test the effects of post-thaw storage time and temperature on boar sperm survival. Semen ejaculates from five Landrace boars (one ejaculate per boar) were collected and frozen following a 0.5 mL-straw protocol. Straws from the five boars were thawed and diluted 1:1 (v:v) in BTS. The frozen-thawed semen samples were aliquoted into three parts and respectively stored at 5 °C, 17 °C, and 37 °C for up to 6 h. At 0.5, 2, and 6 h of storage, sperm motility, viability, mitochondrial membrane potential, and intracellular reactive oxygen species (ROS) levels and apoptotic changes were measured. Antioxidant and oxidant levels were tested in boar sperm (SPZ) and their surrounding environment (SN) at each timepoint. The results showed significant effects of post-thaw storage time and temperature and an impact on boar sperm quality (total and progressive motility, VCL, viability, acrosome integrity), early and late sperm apoptotic changes, and changes in MDA levels in SPZ and SN. Compared to storage at 5 °C and 37 °C, frozen-thawed semen samples stored at 17 °C displayed better sperm quality, less apoptotic levels, and lower levels of SPZ MDA and SN MDA. Notably, post-thaw storage at 17 °C extended boar sperm lifespan up to 6 h without obvious reduction in sperm quality. In conclusion, storage of frozen-thawed boar semen at 17 °C preserves sperm quality for up to 6 h, which facilitates the use of cryopreserved boar semen for field artificial insemination.
Keywords: apoptosis; boar semen cryopreservation; oxidative stress; post-thaw storage; sperm survival.
Conflict of interest statement
Author Shuaibiao Wang was employed by the company DanAg Agritech Consulting (Zhengzhou) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; the collection, analysis, or interpretation of data; writing the manuscript; or the decision to publish the results.
Figures



Similar articles
-
Cryoprotectant effects of natural honey on spermatozoa quality of pre-freezing and frozen-thawed boar semen.J Anim Sci. 2023 Jan 3;101:skac384. doi: 10.1093/jas/skac384. J Anim Sci. 2023. PMID: 36409528 Free PMC article.
-
Post-thaw sperm characteristics following long-term storage of boar semen in liquid nitrogen.Anim Reprod Sci. 2014 Jun 30;147(3-4):119-27. doi: 10.1016/j.anireprosci.2014.04.010. Epub 2014 Apr 30. Anim Reprod Sci. 2014. PMID: 24819551
-
Implications of the pH and temperature of diluted, cooled boar semen on fresh and frozen-thawed sperm motility characteristics.Theriogenology. 2010 Oct 15;74(7):1304-10. doi: 10.1016/j.theriogenology.2010.04.030. Epub 2010 May 21. Theriogenology. 2010. PMID: 20494424
-
Fertility of boar semen cryopreserved in extender supplemented with butylated hydroxytoluene.Theriogenology. 2015 Feb;83(3):307-13. doi: 10.1016/j.theriogenology.2014.07.045. Epub 2014 Oct 30. Theriogenology. 2015. PMID: 25468554 Review.
-
Strategies to improve the fertility of frozen-thawed boar semen for artificial insemination.Soc Reprod Fertil Suppl. 2006;62:261-75. Soc Reprod Fertil Suppl. 2006. PMID: 16866323 Review.
Cited by
-
Boar-to-Boar Variations in Quality Characteristics of Sperm from Different Ejaculates Following Freezing-Thawing.Cells. 2025 Feb 2;14(3):212. doi: 10.3390/cells14030212. Cells. 2025. PMID: 39937003 Free PMC article.
-
Phytochemical Screening, Antioxidant Effect and Sperm Quality of the Bomba ceiba Stamen Extracts on Charolais Cattle Sperm Induced by Ferrous Sulfate.Plants (Basel). 2024 Mar 26;13(7):960. doi: 10.3390/plants13070960. Plants (Basel). 2024. PMID: 38611489 Free PMC article.
-
Integrating the milk microbiome signatures in mastitis: milk-omics and functional implications.World J Microbiol Biotechnol. 2025 Jan 18;41(2):41. doi: 10.1007/s11274-024-04242-1. World J Microbiol Biotechnol. 2025. PMID: 39826029 Free PMC article. Review.
-
Protective Effects of Betaine on Boar Sperm Quality during Liquid Storage and Transport.Animals (Basel). 2024 Sep 19;14(18):2711. doi: 10.3390/ani14182711. Animals (Basel). 2024. PMID: 39335300 Free PMC article.
-
Effects of hesperidin and ascorbic acid combination on boar sperm quality during cryopreservation.Front Vet Sci. 2025 Jul 4;12:1573983. doi: 10.3389/fvets.2025.1573983. eCollection 2025. Front Vet Sci. 2025. PMID: 40687084 Free PMC article.
References
-
- Masuda Y., Kheawkanha T., Nagahama A., Kawasaki K., Konno T., Yamanaka K., Tatemoto H. Antifreeze protein type III addition to freezing extender comprehensively improves post-thaw sperm properties in Okinawan native Agu pig. Anim. Reprod. Sci. 2023;252:107232. doi: 10.1016/j.anireprosci.2023.107232. - DOI - PubMed
-
- Pedrosa A.C., Andrade Torres M., Vilela Alkmin D., Pinzon J.E.P., Kitamura Martins S.M.M., Coelho da Silveira J., Furugen Cesar de Andrade A. Spermatozoa and seminal plasma small extracellular vesicles miRNAs as biomarkers of boar semen cryotolerance. Theriogenology. 2021;174:60–72. doi: 10.1016/j.theriogenology.2021.07.022. - DOI - PubMed
Grants and funding
- 32002355/National Natural Science Foundation of China
- BK20200933/Jiangsu Provincial Natural Science Foundation of China
- JSSCTD202224/Jiangsu Shuangchuang Innovation Group Project
- 2020SY003YD12/Jiangsu Province Modern Agriculture Development Project
- PAPD/A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions
LinkOut - more resources
Full Text Sources
Miscellaneous

