Mass Spectrometry Rearrangement Ions and Metabolic Pathway-Based Discovery of Indole Derivatives during the Aging Process in Citrus reticulata 'Chachi'
- PMID: 38201037
- PMCID: PMC10778486
- DOI: 10.3390/foods13010008
Mass Spectrometry Rearrangement Ions and Metabolic Pathway-Based Discovery of Indole Derivatives during the Aging Process in Citrus reticulata 'Chachi'
Abstract
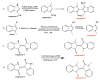
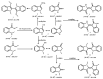
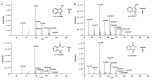
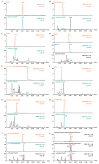
The rapid analysis and characterization of compounds using mass spectrometry (MS) may overlook trace compounds. Although targeted analysis methods can significantly improve detection sensitivity, it is hard to discover novel scaffold compounds in the trace. This study developed a strategy for discovering trace compounds in the aging process of traditional Chinese medicine based on MS fragmentation and known metabolic pathways. Specifically, we found that the characteristic component of C. reticulata 'Chachi', methyl N-methyl anthranilate (MMA), fragmented in electrospray ionization coupled with collision-induced dissociation (CID) to produce the rearrangement ion 3-hydroxyindole, which was proven to exist in trace amounts in C. reticulata 'Chachi' based on comparison with the reference substance using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Combining the known metabolic pathways of 3-hydroxyindole and the possible methylation reactions that may occur during aging, a total of 10 possible indole derivatives were untargeted predicted. These compounds were confirmed to originate from MMA using purchased or synthesized reference substances, all of which were detected in C. reticulata 'Chachi' through LC-MS/MS, achieving trace compound analysis from untargeted to targeted. These results may contribute to explaining the aging mechanism of C. reticulata 'Chachi', and the strategy of using the CID-induced special rearrangement ion-binding metabolic pathway has potential application value for discovering trace compounds.
Keywords: 3-hydroxyindole; Citrus reticulata ‘Chachi’; indole derivatives; mass spectrometry; methyl N-methyl anthranilate.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Rapid Discrimination of Citrus reticulata 'Chachi' by Electrospray Ionization-Ion Mobility-High-Resolution Mass Spectrometry.Molecules. 2021 Nov 20;26(22):7015. doi: 10.3390/molecules26227015. Molecules. 2021. PMID: 34834108 Free PMC article.
-
Discrimination of Citrus reticulata Blanco and Citrus reticulata 'Chachi' as well as the Citrus reticulata 'Chachi' within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach.J Pharm Biomed Anal. 2019 Jul 15;171:218-231. doi: 10.1016/j.jpba.2019.03.056. Epub 2019 Mar 29. J Pharm Biomed Anal. 2019. PMID: 31072532
-
Discrimination of Citrus reticulata Blanco and Citrus reticulata 'Chachi' by gas chromatograph-mass spectrometry based metabolomics approach.Food Chem. 2016 Dec 1;212:123-7. doi: 10.1016/j.foodchem.2016.05.141. Epub 2016 May 24. Food Chem. 2016. PMID: 27374515
-
"Polymeromics": Mass spectrometry based strategies in polymer science toward complete sequencing approaches: a review.Anal Chim Acta. 2014 Jan 15;808:56-69. doi: 10.1016/j.aca.2013.10.027. Epub 2013 Oct 21. Anal Chim Acta. 2014. PMID: 24370093 Review.
-
Critical practical aspects in the application of liquid chromatography-mass spectrometric studies for the characterization of impurities and degradation products.J Pharm Biomed Anal. 2014 Jan;87:191-217. doi: 10.1016/j.jpba.2013.04.027. Epub 2013 Apr 28. J Pharm Biomed Anal. 2014. PMID: 23706957 Review.
References
-
- Visconti G., de Figueiredo M., Strassel O., Boccard J., Vuilleumier N., Jaques D., Ponte B., Rudaz S. Multitargeted Internal Calibration for the Quantification of Chronic Kidney Disease-Related Endogenous Metabolites Using Liquid Chromatography–Mass Spectrometry. Anal. Chem. 2023;95:13546–13554. doi: 10.1021/acs.analchem.3c02069. - DOI - PMC - PubMed
-
- Xue G., Su S., Yan P., Shang J., Wang J., Yan C., Li J., Wang Q., Xiong X., Xu H. Integrative analyses of widely targeted metabolomic profiling and derivatization-based LC-MS/MS reveals metabolic changes of Zingiberis Rhizoma and its processed products. Food Chem. 2022;389:133068. doi: 10.1016/j.foodchem.2022.133068. - DOI - PubMed
-
- Custodio-Mendoza J.A., Sendón R., de Quirós A.R.-B., Lorenzo R.A., Carro A.M. Development of a QuEChERS method for simultaneous analysis of 3-Monochloropropane-1,2-diol monoesters and Glycidyl esters in edible oils and margarine by LC-APCI-MS/MS. Anal. Chim. Acta. 2023;1239:340712. doi: 10.1016/j.aca.2022.340712. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

