Prospective Randomized, Double-Blind, Placebo-Controlled Study of a Standardized Oral Pomegranate Extract on the Gut Microbiome and Short-Chain Fatty Acids
- PMID: 38201042
- PMCID: PMC10778484
- DOI: 10.3390/foods13010015
Prospective Randomized, Double-Blind, Placebo-Controlled Study of a Standardized Oral Pomegranate Extract on the Gut Microbiome and Short-Chain Fatty Acids
Abstract
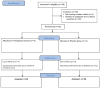
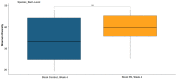
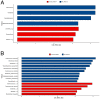
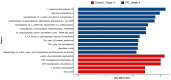
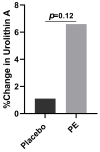
Punica granatum L., commonly known as the pomegranate, is an abundant source of polyphenols, including hydrolyzable ellagitannins, ellagic acid, anthocyanins, and other bioactive phytochemicals shown to be effective in defending against oxidative stress, and has immunomodulatory activities. Ellagitannins, and their hydrolyzed product ellagic acid, interact with the gut microbiota to yield secondary metabolites known as urolithins that may have health benefits. The objective of this study was to determine the effects of supplementation with a standardized punicalagin-enriched pomegranate extract, Pomella® (250 mg), on the gut microbiome, circulating short-chain fatty acids, and gut microbial-derived ellagitannin metabolite urolithins. A randomized, double-blind, placebo-controlled study was conducted over 4 weeks on healthy volunteers aged 25-55 years. Subjects were randomly assigned to receive either an oral supplement containing 75 mg of punicalagin or an oral placebo. Stool sample collection and venipuncture were performed to analyze the gut microbiome, SCFAs, and urolithin. There was no significant change in the gut microbial diversity in both cohorts after 4 weeks of intervention, but there was a significantly increased relative abundance of Coprococcus eutectus, Roseburia faecis, Roseburia inullnivorans, Ruminococcus bicirculans, Ruminococcus calidus, and Faecalibacterium prausnitzii. Pomegranate extract (PE) supplementation led to the augmentation of circulating propionate levels (p = 0.02) and an increasing trend for acetate levels (p = 0.12). The pomegranate extract (PE) supplementation group had an increased level of circulating urolithins compared to the placebo group (6.6% vs. 1.1%, p = 0.13). PE supplementation correlated with shifts in the gut microbiome and with higher circulating levels of propionate and acetate. Further studies should explore the implications in larger cohorts and over a longer duration.
Keywords: Pomella; ellagitannins; gut health; gut microbiome; pomegranate; punicalagin; short-chain fatty acids; urolithins.
Conflict of interest statement
Mincy Chakkalakal, Adrianne Pan, Dawnica Nadora, Mildred Min, Ashley Dumont, Waqas A. Burney, and Cindy J. Chambers have no interests to declare. Raja K. Sivamani is a scientific advisor for LearnHealth, Arbonne, and Codex Labs and has served as a consultant or speaker for Burt’s Bees, Novozymes, Biogena, Novartis, Sanofi, Bristol Myers Squibb, Pfizer, Nutrafol, Galderma, Novartis, Bristol Myer Squibb, Abbvie, Leo, UCB, Sun, and Regeneron Pharmaceuticals.
Figures

References
-
- Lavoro A., Falzone L., Gattuso G., Salemi R., Cultrera G., Leone G.M., Scandurra G., Candido S., Libra M. Pomegranate: A promising avenue against the most common chronic diseases and their associated risk factors (Review) Int. J. Funct. Nutr. 2021;2:6. doi: 10.3892/ijfn.2021.16. - DOI
-
- Neyrinck A.M., Van Hee V.F., Bindels L.B., De Backer F., Cani P.D., Delzenne N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013;109:802–809. doi: 10.1017/S0007114512002206. - DOI - PubMed
-
- Singh A., D’Amico D., Andreux P.A., Dunngalvin G., Kern T., Blanco-Bose W., Auwerx J., Aebischer P., Rinsch C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022;76:297–308. doi: 10.1038/s41430-021-00950-1. - DOI - PMC - PubMed

