Exploring In Vitro the Combination of Cistus × incanus L. and Castanea sativa Mill. Extracts as Food Supplement Ingredients against H. pylori Infection
- PMID: 38201068
- PMCID: PMC10778332
- DOI: 10.3390/foods13010040
Exploring In Vitro the Combination of Cistus × incanus L. and Castanea sativa Mill. Extracts as Food Supplement Ingredients against H. pylori Infection
Abstract
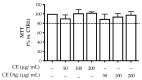
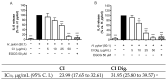
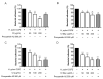
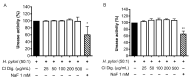
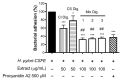
Cistus spp. have been traditionally used for inflammatory and infectious disorders, including gastrointestinal ailments, in the Mediterranean area. Among them, Cistus × incanus L. is one of the most frequently cited species in the literature for a variety of biological activities which include inflammatory diseases. Cistus spp. aerial parts are rich in polyphenols such as condensed and hydrolysable tannins, procyanidins, and flavonoids, which show gastroprotective activities. The purpose of the present study is to investigate the biological activities of a hydroalcoholic extract from Cistus × incanus L. aerial parts in gastric epithelial cells (GES-1) infected with H. pylori. The extracts inhibited IL-8 and NF-κB induced by H. pylori and showed antibacterial activity after simulated digestion. Since our previous paper reported interesting results on the ability of Castanea sativa Mill. leaf extract to decrease inflammatory conditions in H. pylori-infected gastric cells, the combination of Castanea sativa and Cistus × incanus extracts was also investigated, showing strong anti-inflammatory activity and inhibition of bacterial adhesion. This association of botanicals is proposed herein as a novel food supplement capable of counteracting gastric inflammatory conditions.
Keywords: Cistus; Cistus × incanus; Helicobacter pylori; gastritis; inflammation.
Conflict of interest statement
G.N. and S.F.V. are, respectively, the scientific director and the R&D officer responsible from EPO s.r.l. However, this paper does not necessarily reflect the company’s views of its future policy on this area. The authors declare no conflict of interest.
Figures


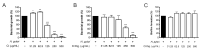
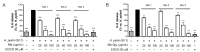

Similar articles
-
Ellagitannins from Castanea sativa Mill. Leaf Extracts Impair H. pylori Viability and Infection-Induced Inflammation in Human Gastric Epithelial Cells.Nutrients. 2023 Mar 21;15(6):1504. doi: 10.3390/nu15061504. Nutrients. 2023. PMID: 36986236 Free PMC article.
-
Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics.Antioxidants (Basel). 2020 Mar 1;9(3):202. doi: 10.3390/antiox9030202. Antioxidants (Basel). 2020. PMID: 32121584 Free PMC article.
-
The Polyphenolic Composition of Cistus incanus Herbal Tea and Its Antibacterial and Anti-adherent Activity against Streptococcus mutans.Planta Med. 2015 Dec;81(18):1727-35. doi: 10.1055/s-0035-1557822. Epub 2015 Aug 20. Planta Med. 2015. PMID: 26291656
-
Medicinal plant activity on Helicobacter pylori related diseases.World J Gastroenterol. 2014 Aug 14;20(30):10368-82. doi: 10.3748/wjg.v20.i30.10368. World J Gastroenterol. 2014. PMID: 25132753 Free PMC article. Review.
-
Beneficial Effects of Castanea sativa Wood Extract on the Human Body and Possible Food and Pharmaceutical Applications.Plants (Basel). 2024 Mar 22;13(7):914. doi: 10.3390/plants13070914. Plants (Basel). 2024. PMID: 38611444 Free PMC article. Review.
Cited by
-
Biology, Antioxidant Activity, and Therapeutic Potential of Cistus sp.-A Comprehensive Review.Int J Mol Sci. 2025 Jul 3;26(13):6400. doi: 10.3390/ijms26136400. Int J Mol Sci. 2025. PMID: 40650177 Free PMC article. Review.
-
Antitumor Effects of Resveratrol Opposing Mechanisms of Helicobacter pylori in Gastric Cancer.Nutrients. 2024 Jul 4;16(13):2141. doi: 10.3390/nu16132141. Nutrients. 2024. PMID: 38999888 Free PMC article. Review.
References
-
- Tomou E.-M., Lytra K., Rallis S., Tzakos A.G., Skaltsa H. An updated review of genus Cistus L. since 2014: Traditional uses, phytochemistry, and pharmacological properties. Phytochem. Rev. 2022;21:2049–2087. doi: 10.1007/s11101-022-09827-y. - DOI
-
- Viapiana A., Konopacka A., Waleron K., Wesolowski M. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crops Prod. 2017;107:297–304. doi: 10.1016/j.indcrop.2017.05.066. - DOI
-
- Mansoor K.A., Matalka K.Z., Qa’dan F.S., Awad R., Schmidt M. Two new proanthocyanidin trimers isolated from Cistus incanus L. demonstrate potent anti-inflammatory activity and selectivity to cyclooxygenase isoenzymes inhibition. Nat. Prod. Res. 2016;30:1919–1926. doi: 10.1080/14786419.2015.1089242. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

