Acetylcholinesterase- and Butyrylcholinesterase-Based Biosensors for the Detection of Quaternary Ammonium Biocides in Food Industry
- PMID: 38201162
- PMCID: PMC10779051
- DOI: 10.3390/foods13010133
Acetylcholinesterase- and Butyrylcholinesterase-Based Biosensors for the Detection of Quaternary Ammonium Biocides in Food Industry
Abstract
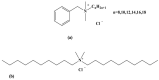
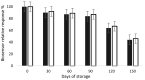
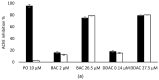
A sensitive and robust electrochemical cholinesterase-based sensor was developed to detect the quaternary ammonium (QAs) biocides most frequently found in agri-food industry wash waters: benzalkonium chloride (BAC) and didecyldimethylammonium chloride (DDAC). To reach the maximum residue limit of 28 nM imposed by the European Union (EU), two types of cholinesterases were tested, acetylcholinesterase (AChE, from Drosophila melanogaster) and butyrylcholinesterase (BChE, from horse serum). The sensors were designed by entrapping AChE or BChE on cobalt phthalocyanine-modified screen-printed carbon electrodes. The limits of detection (LOD) of the resulting biosensors were 38 nM for DDAC and 320 nM for BAC, using, respectively, AChE and BChE. A simple solid-phase extraction step was used to concentrate the samples before biosensor analysis, allowing for the accurate determination of DDAC and BAC in tap water with limits of quantification (LOQ) as low as 2.7 nM and 5.3 nM, respectively. Additional assays demonstrated that the use of a phosphotriesterase enzyme allows for the total removal of interferences due to the possible presence of organophosphate insecticides in the sample. The developed biosensors were shown to be stable during 3 months storage at 4 °C.
Keywords: biocides; biosensor; cholinesterases; phosphotriesterase; quaternary ammoniums; screen-printed electrodes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chauret C.P. Encyclopedia of Food Microbiology. 2nd ed. Academic Press; Oxford, UK: 2014. Sanitization; pp. 360–364.
LinkOut - more resources
Full Text Sources
Miscellaneous

