Biomarkers of Affective Dysregulation Associated with In Utero Exposure to EtOH
- PMID: 38201206
- PMCID: PMC10778368
- DOI: 10.3390/cells13010002
Biomarkers of Affective Dysregulation Associated with In Utero Exposure to EtOH
Abstract
Introduction: Children with fetal alcohol spectrum disorders (FASD) exhibit behavioral and affective dysregulation, including hyperactivity and depression. The mechanisms are not known, but they could conceivably be due to postnatal social or environmental factors. However, we postulate that, more likely, the affective dysregulation is associated with the effects of EtOH exposure on the development of fetal serotonergic (5-HT) and/or dopaminergic (DA) pathways, i.e., pathways that in postnatal life are believed to regulate mood. Many women who use alcohol (ethanol, EtOH) during pregnancy suffer from depression and take selective serotonin reuptake inhibitors (SSRIs), which might influence these monoaminergic pathways in the fetus. Alternatively, monoaminergic pathway abnormalities might reflect a direct effect of EtOH on the fetal brain. To distinguish between these possibilities, we measured their expressions in fetal brains and in fetal brain-derived exosomes (FB-Es) isolated from the mothers' blood. We hypothesized that maternal use of EtOH and/or SSRIs during pregnancy would be associated with impaired fetal neural development, detectable as abnormal levels of monoaminergic and apoptotic biomarkers in FB-Es.
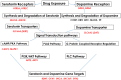
Methods: Fetal brain tissues and maternal blood were collected at 9-23 weeks of pregnancy. EtOH groups were compared with unexposed controls matched for gestational age (GA). The expression of 84 genes associated with the DA and 5-HT pathways was analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) on microarrays. FB-Es also were assayed for serotonin transporter protein (SERT) and brain-derived neurotrophic factor (BDNF) by enzyme-linked immunosorbent assay (ELISA).
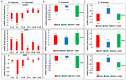
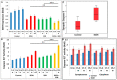
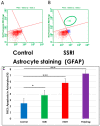
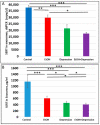
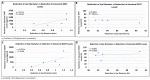
Results: Six EtOH-exposed human fetal brain samples were compared to SSRI- or polydrug-exposed samples and to unexposed controls. EtOH exposure was associated with significant upregulation of DA receptor D3 and 5-HT receptor HTR2C, while HTR3A was downregulated. Monoamine oxidase A (MAOA), MAOB, the serine/threonine kinase AKT3, and caspase-3 were upregulated, while mitogen-activated protein kinase 1 (MAPK1) and AKT2 were downregulated. ETOH was associated with significant upregulation of the DA transporter gene, while SERT was downregulated. There were significant correlations between EtOH exposure and (a) caspase-3 activation, (b) reduced SERT protein levels, and (c) reduced BDNF levels. SSRI exposure independently increased caspase-3 activity and downregulated SERT and BDNF. Early exposure to EtOH and SSRI together was associated synergistically with a significant upregulation of caspase-3 and a significant downregulation of SERT and BDNF. Reduced SERT and BDNF levels were strongly correlated with a reduction in eye diameter, a somatic manifestation of FASD.
Conclusions: Maternal use of EtOH and SSRI during pregnancy each was associated with changes in fetal brain monoamine pathways, consistent with potential mechanisms for the affective dysregulation associated with FASD.
Keywords: FAS; FASD; biomarkers; brain development; depression; dopamine receptors; exosomes; serotonin receptors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Prenatal Alcohol Exposure Inhibits Transient Expression of Autophagy and Synaptic Proteins in Developing Brain.Obstet Gynecol Res. 2025;8(1):10-24. doi: 10.26502/ogr0173. Epub 2025 Jan 22. Obstet Gynecol Res. 2025. PMID: 40084086 Free PMC article.
-
Effects of In Utero EtOH Exposure on 18S Ribosomal RNA Processing: Contribution to Fetal Alcohol Spectrum Disorder.Int J Mol Sci. 2023 Sep 5;24(18):13714. doi: 10.3390/ijms241813714. Int J Mol Sci. 2023. PMID: 37762017 Free PMC article.
-
Molecular Markers in Maternal Blood Exosomes Allow Early Detection of Fetal Alcohol Spectrum Disorders.Int J Mol Sci. 2022 Dec 21;24(1):135. doi: 10.3390/ijms24010135. Int J Mol Sci. 2022. PMID: 36613580 Free PMC article.
-
Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review.
-
Development of the Placenta and Brain Are Affected by Selective Serotonin Reuptake Inhibitor Exposure During Critical Periods.Adv Exp Med Biol. 2023;1428:179-198. doi: 10.1007/978-3-031-32554-0_8. Adv Exp Med Biol. 2023. PMID: 37466774 Review.
Cited by
-
Prenatal Alcohol Exposure Inhibits Transient Expression of Autophagy and Synaptic Proteins in Developing Brain.Obstet Gynecol Res. 2025;8(1):10-24. doi: 10.26502/ogr0173. Epub 2025 Jan 22. Obstet Gynecol Res. 2025. PMID: 40084086 Free PMC article.
-
Fetal Brain-Derived Exosomal miRNAs from Maternal Blood: Potential Diagnostic Biomarkers for Fetal Alcohol Spectrum Disorders (FASDs).Int J Mol Sci. 2024 May 27;25(11):5826. doi: 10.3390/ijms25115826. Int J Mol Sci. 2024. PMID: 38892014 Free PMC article.
-
Prenatal Opioid and Alcohol Exposures: Association with Altered Placental Serotonin Transporter Structure and/or Expression.Int J Mol Sci. 2024 Oct 28;25(21):11570. doi: 10.3390/ijms252111570. Int J Mol Sci. 2024. PMID: 39519122 Free PMC article.
-
Serum untargeted metabolomics alterations in systemic lupus erythematosus patients with elevated serum ferritin.Sci Rep. 2025 Jul 15;15(1):25481. doi: 10.1038/s41598-025-06170-y. Sci Rep. 2025. PMID: 40664755 Free PMC article.
-
In Utero Alcohol and Tobacco Exposure, Maternal Depression, And Maternal Obesity Are Associated with Impaired Oligodendrocyte Differentiation in The Developing Brain.Obstet Gynecol Res. 2025;8(1):1-9. doi: 10.26502/ogr0172. Epub 2025 Jan 15. Obstet Gynecol Res. 2025. PMID: 40061215 Free PMC article.
References
-
- Mutch R.C., Jones H.M., Bower C., Watkins R.E. Fetal Alcohol Spectrum Disorders: Using Knowledge, Attitudes and Practice of Justice Professionals to Support their Educational Needs. J. Popul. Ther. Clin. Pharmacol. 2016;23:e77–e89. - PubMed
-
- Sampson P.D., Streissguth A.P., Bookstein F.L., Little R.E., Clarren S.K., Dehaene P., Hanson J.W., Graham J.M., Jr. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

