Multiple-Pathway Synergy Alters Steroidogenesis and Spermatogenesis in Response to an Immunocastration Vaccine in Goat
- PMID: 38201210
- PMCID: PMC10778245
- DOI: 10.3390/cells13010006
Multiple-Pathway Synergy Alters Steroidogenesis and Spermatogenesis in Response to an Immunocastration Vaccine in Goat
Abstract
Background: Animal reproduction performance is crucial in husbandry. Immunocastrated animals serve as an ideal animal model for studying testicular function. During androgen suppression, the testis undergoes dramatic developmental and structural changes, including the inhibition of hormone secretion and spermatogenesis.
Methods: To characterize this process, we investigated the effects of castration using a recombinant B2L and KISS1 DNA vaccine, and then identified functional genes in the testes of Yiling goats using RNA-seq and WGS. The experimental animals were divided into three groups: the PVAX-asd group (control), PBK-asd-immunized group, and surgically castrated group.
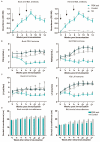
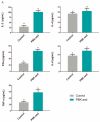
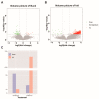
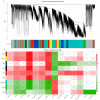
Results: The results demonstrated that the administration of the recombinant PBK-asd vaccine in goats elicited a significant antibody response, and reduced serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH), resulting in smaller scrotal circumferences and decreased sexual desire compared to the control group. In addition, RNA transcriptome sequencing (RNA-seq) analysis of the testes revealed that the biological processes after immunocastration mainly focused on the regulation of cell matrix adhesion, histone acetylation, negative regulation of developmental processes, apoptosis, and activation of the complement system and the thrombin cascade reaction system. Then, we integrated the whole-genome sequencing and testis transcriptome, and identified several candidate genes (FGF9, FST, KIT, TH, TCP1, PLEKHA1, TMEM119, ESR1, TIPARP, LEP) that influence steroidogenesis secretion and spermatogenesis.
Conclusions: Multiple pathways and polygenic co-expression participate in the response to castration vaccines, altering hormone secretion and spermatogenesis. Taken together, our atlas of the immunocastration goat testis provides multiple insights into the developmental changes and key factors accompanying androgen suppression, and thus may contribute to understanding the genetic mechanism of testis function. Joint analysis of whole genome sequencing and RNA-seq enables reliable screening of candidate genes, benefiting future genome-assisted breeding of goats.
Keywords: DNA vaccine; goat; immunocastration; testis function; transcriptome; whole gene sequencing.
Conflict of interest statement
All authors declare no conflict of interest regarding any matter in this study.
Figures



Similar articles
-
RNA-Seq reveals ACTH-induced steroid hormone pathway participating in goat adrenal gland response to castration.Sci Rep. 2023 Aug 28;13(1):14025. doi: 10.1038/s41598-023-41016-5. Sci Rep. 2023. PMID: 37640763 Free PMC article.
-
Transcriptomic Analysis of the Developing Testis and Spermatogenesis in Qianbei Ma Goats.Genes (Basel). 2023 Jun 25;14(7):1334. doi: 10.3390/genes14071334. Genes (Basel). 2023. PMID: 37510239 Free PMC article.
-
Multipathway synergy promotes testicular transition from growth to spermatogenesis in early-puberty goats.BMC Genomics. 2020 May 25;21(1):372. doi: 10.1186/s12864-020-6767-x. BMC Genomics. 2020. PMID: 32450814 Free PMC article.
-
The role of GnRH in Tibetan male sheep and goat reproduction.Reprod Domest Anim. 2023 Sep;58(9):1179-1187. doi: 10.1111/rda.14432. Epub 2023 Jul 26. Reprod Domest Anim. 2023. PMID: 37492901 Review.
-
Hormones and hormonal target cells in the testis.Andrologia. 1976;8(3):195-202. doi: 10.1111/j.1439-0272.1976.tb02135.x. Andrologia. 1976. PMID: 187086 Review.
Cited by
-
Preparation of two kinds of immunocastration vaccines and their immune effects on male goats.Anim Biosci. 2025 Jul;38(7):1411-1421. doi: 10.5713/ab.24.0811. Epub 2025 Apr 11. Anim Biosci. 2025. PMID: 40241592 Free PMC article.
References
-
- Larson G., Piperno D.R., Allaby R.G., Purugganan M.D., Andersson L., Arroyo-Kalin M., Barton L., Vigueira C.C., Denham T., Dobney K., et al. Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. USA. 2014;111:6139–6146. doi: 10.1073/pnas.1323964111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

