WAVE2 Is a Vital Regulator in Myogenic Differentiation of Progenitor Cells through the Mechanosensitive MRTFA-SRF Axis
- PMID: 38201213
- PMCID: PMC10778525
- DOI: 10.3390/cells13010009
WAVE2 Is a Vital Regulator in Myogenic Differentiation of Progenitor Cells through the Mechanosensitive MRTFA-SRF Axis
Abstract
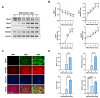
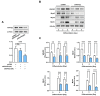
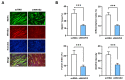
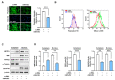
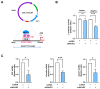
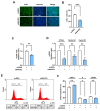
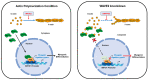
Skeletal myogenesis is an intricate process involving the differentiation of progenitor cells into myofibers, which is regulated by actin cytoskeletal dynamics and myogenic transcription factors. Although recent studies have demonstrated the pivotal roles of actin-binding proteins (ABPs) as mechanosensors and signal transducers, the biological significance of WAVE2 (Wiskott-Aldrich syndrome protein family member 2), an ABP essential for actin polymerization, in myogenic differentiation of progenitor cells has not been investigated. Our study provides important insights into the regulatory roles played by WAVE2 in the myocardin-related transcription factor A (MRTFA)-serum response factor (SRF) signaling axis and differentiation of myoblasts. We demonstrate that WAVE2 expression is induced during myogenic differentiation and plays a pivotal role in actin cytoskeletal remodeling in C2C12 myoblasts. Knockdown of WAVE2 in C2C12 cells reduced filamentous actin levels, increased globular actin accumulation, and impaired the nuclear translocation of MRTFA. Furthermore, WAVE2 depletion in myoblasts inhibited the expression and transcriptional activity of SRF and suppressed cell proliferation in myoblasts. Consequently, WAVE2 knockdown suppressed myogenic regulatory factors (i.e., MyoD, MyoG, and SMYD1) expressions, thereby hindering the differentiation of myoblasts. Thus, this study suggests that WAVE2 is essential for myogenic differentiation of progenitor cells by modulating the mechanosensitive MRTFA-SRF axis.
Keywords: MRTFA; SRF; WAVE2; differentiation; myogenesis; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

