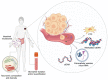
Promising and Minimally Invasive Biomarkers: Targeting Melanoma
- PMID: 38201222
- PMCID: PMC10777980
- DOI: 10.3390/cells13010019
Promising and Minimally Invasive Biomarkers: Targeting Melanoma
Abstract
The therapeutic landscape of malignant melanoma has been radically reformed in recent years, with novel treatments emerging in both the field of cancer immunotherapy and signalling pathway inhibition. Large-scale tumour genomic characterization has accurately classified malignant melanoma into four different genomic subtypes so far. Despite this, only somatic mutations in BRAF oncogene, as assessed in tumour biopsies, has so far become a validated predictive biomarker of treatment with small molecule inhibitors. The biology of tumour evolution and heterogeneity has uncovered the current limitations associated with decoding genomic drivers based only on a single-site tumour biopsy. There is an urgent need to develop minimally invasive biomarkers that accurately reflect the real-time evolution of melanoma and that allow for streamlined collection, analysis, and interpretation. These will enable us to face challenges with tumour tissue attainment and process and will fulfil the vision of utilizing "liquid biopsy" to guide clinical decisions, in a manner akin to how it is used in the management of haematological malignancies. In this review, we will summarize the most recent published evidence on the role of minimally invasive biomarkers in melanoma, commenting on their future potential to lead to practice-changing discoveries.
Keywords: circulating melanoma cells; circulating tumour DNA; extracellular vesicles; intestinal microbiome; melanoma; minimally invasive biomarkers; predictive; prognostic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy.Pathology. 2016 Feb;48(2):113-31. doi: 10.1016/j.pathol.2015.12.007. Epub 2016 Jan 18. Pathology. 2016. PMID: 27020384 Free PMC article. Review.
-
Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients.Mol Oncol. 2016 Mar;10(3):450-63. doi: 10.1016/j.molonc.2015.12.008. Epub 2015 Dec 17. Mol Oncol. 2016. PMID: 26778792 Free PMC article. Review.
-
Melanoma subtypes: genomic profiles, prognostic molecular markers and therapeutic possibilities.J Pathol. 2019 Apr;247(5):539-551. doi: 10.1002/path.5213. Epub 2019 Feb 15. J Pathol. 2019. PMID: 30511391 Free PMC article. Review.
-
Liquid biopsy-based analysis by ddPCR and CAPP-Seq in melanoma patients.J Dermatol Sci. 2021 Jun;102(3):158-166. doi: 10.1016/j.jdermsci.2021.04.006. Epub 2021 Apr 30. J Dermatol Sci. 2021. PMID: 34049769
-
Genomic profiling of a dedifferentiated mucosal melanoma following exposure to immunotherapy.Melanoma Res. 2020 Apr;30(2):213-218. doi: 10.1097/CMR.0000000000000636. Melanoma Res. 2020. PMID: 31425481
Cited by
-
Detection of multiple activating NRAS variants under BRAF/MEK-inhibitor therapy in BRAF positive malignant melanoma using liquid biopsy.JAAD Case Rep. 2024 Jul 23;52:4-7. doi: 10.1016/j.jdcr.2024.06.036. eCollection 2024 Oct. JAAD Case Rep. 2024. PMID: 39282524 Free PMC article. No abstract available.
-
Morphological and Immunohistochemical Aspects with Prognostic Implications and Therapeutic Targets of Primary Sinonasal Mucosal Melanoma: A Retrospective Study.Cancers (Basel). 2024 Aug 16;16(16):2863. doi: 10.3390/cancers16162863. Cancers (Basel). 2024. PMID: 39199634 Free PMC article.
-
Liquid biopsy as a potential tool for diagnosis and clinical monitoring of cutaneous and uveal melanoma.J Liq Biopsy. 2025 Jun 14;9:100306. doi: 10.1016/j.jlb.2025.100306. eCollection 2025 Sep. J Liq Biopsy. 2025. PMID: 40585187 Free PMC article. Review.
-
Advancements and Challenges in Personalized Therapy for BRAF-Mutant Melanoma: A Comprehensive Review.J Clin Med. 2024 Sep 12;13(18):5409. doi: 10.3390/jcm13185409. J Clin Med. 2024. PMID: 39336897 Free PMC article. Review.
References
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
-
- Dummer R., Long G.V., Robert C., Tawbi H.A., Flaherty K.T., Ascierto P.A., Nathan P.D., Rutkowski P., Leonov O., Dutriaux C., et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022;40:1428–1438. doi: 10.1200/JCO.21.01601. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials