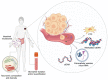
Promising and Minimally Invasive Biomarkers: Targeting Melanoma
- PMID: 38201222
- PMCID: PMC10777980
- DOI: 10.3390/cells13010019
Promising and Minimally Invasive Biomarkers: Targeting Melanoma
Abstract
The therapeutic landscape of malignant melanoma has been radically reformed in recent years, with novel treatments emerging in both the field of cancer immunotherapy and signalling pathway inhibition. Large-scale tumour genomic characterization has accurately classified malignant melanoma into four different genomic subtypes so far. Despite this, only somatic mutations in BRAF oncogene, as assessed in tumour biopsies, has so far become a validated predictive biomarker of treatment with small molecule inhibitors. The biology of tumour evolution and heterogeneity has uncovered the current limitations associated with decoding genomic drivers based only on a single-site tumour biopsy. There is an urgent need to develop minimally invasive biomarkers that accurately reflect the real-time evolution of melanoma and that allow for streamlined collection, analysis, and interpretation. These will enable us to face challenges with tumour tissue attainment and process and will fulfil the vision of utilizing "liquid biopsy" to guide clinical decisions, in a manner akin to how it is used in the management of haematological malignancies. In this review, we will summarize the most recent published evidence on the role of minimally invasive biomarkers in melanoma, commenting on their future potential to lead to practice-changing discoveries.
Keywords: circulating melanoma cells; circulating tumour DNA; extracellular vesicles; intestinal microbiome; melanoma; minimally invasive biomarkers; predictive; prognostic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
-
- Dummer R., Long G.V., Robert C., Tawbi H.A., Flaherty K.T., Ascierto P.A., Nathan P.D., Rutkowski P., Leonov O., Dutriaux C., et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022;40:1428–1438. doi: 10.1200/JCO.21.01601. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials