Fully Human Herpesvirus-Specific Neutralizing IgG Antibodies Generated by EBV Immortalization of Splenocytes-Derived from Immunized Humanized Mice
- PMID: 38201224
- PMCID: PMC10778511
- DOI: 10.3390/cells13010020
Fully Human Herpesvirus-Specific Neutralizing IgG Antibodies Generated by EBV Immortalization of Splenocytes-Derived from Immunized Humanized Mice
Abstract
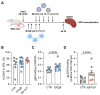
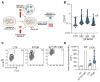
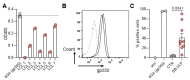
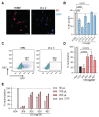
Antiviral neutralizing antibodies (nAbs) are commonly derived from B cells developed in immunized or infected animals and humans. Fully human antibodies are preferred for clinical use as they are potentially less immunogenic. However, the function of B cells varies depending on their homing pattern and an additional hurdle for antibody discovery in humans is the source of human tissues with an immunological microenvironment. Here, we show an efficient method to pharm human antibodies using immortalized B cells recovered from Nod.Rag.Gamma (NRG) mice reconstituting the human immune system (HIS). Humanized HIS mice were immunized either with autologous engineered dendritic cells expressing the human cytomegalovirus gB envelope protein (HCMV-gB) or with Epstein-Barr virus-like particles (EB-VLP). Human B cells recovered from spleen of HIS mice were efficiently immortalized with EBV in vitro. We show that these immortalized B cells secreted human IgGs with neutralization capacities against prototypic HCMV-gB and EBV-gp350. Taken together, we show that HIS mice can be successfully used for the generation and pharming fully human IgGs. This technology can be further explored to generate antibodies against emerging infections for diagnostic or therapeutic purposes.
Keywords: EBV; HCMV; antibodies; dendritic cells; herpesvirus; humanized mice; immortalization; virus-like particles.
Conflict of interest statement
The laboratory of R.S. receives research funding from The Jackson Laboratory for development of humanized mice with improved immunologic functions. The other authors declare no conflict of interest.
Figures
Similar articles
-
Novel Epstein-Barr virus-like particles incorporating gH/gL-EBNA1 or gB-LMP2 induce high neutralizing antibody titers and EBV-specific T-cell responses in immunized mice.Oncotarget. 2017 Mar 21;8(12):19255-19273. doi: 10.18632/oncotarget.13770. Oncotarget. 2017. PMID: 27926486 Free PMC article.
-
Vesicular Stomatitis Virus-Based Epstein-Barr Virus Vaccines Elicit Strong Protective Immune Responses.J Virol. 2022 May 11;96(9):e0033622. doi: 10.1128/jvi.00336-22. Epub 2022 Apr 11. J Virol. 2022. PMID: 35404082 Free PMC article.
-
Repertoire characterization and validation of gB-specific human IgGs directly cloned from humanized mice vaccinated with dendritic cells and protected against HCMV.PLoS Pathog. 2020 Jul 15;16(7):e1008560. doi: 10.1371/journal.ppat.1008560. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32667948 Free PMC article.
-
High Epstein-Barr Virus Load and Genomic Diversity Are Associated with Generation of gp350-Specific Neutralizing Antibodies following Acute Infectious Mononucleosis.J Virol. 2016 Dec 16;91(1):e01562-16. doi: 10.1128/JVI.01562-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27733645 Free PMC article.
-
Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases.Front Immunol. 2021 Oct 8;12:734471. doi: 10.3389/fimmu.2021.734471. eCollection 2021. Front Immunol. 2021. PMID: 34691042 Free PMC article. Review.
References
-
- Schampera M.S., Schweinzer K., Abele H., Kagan K.O., Klein R., Rettig I., Jahn G., Hamprecht K. Comparison of cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: Impact of CMV IgG normalization. J. Clin. Virol. 2017;90:40–45. doi: 10.1016/j.jcv.2017.03.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

