Aberrations in Cell Signaling Quantified in Diabetic Murine Globes after Injury
- PMID: 38201230
- PMCID: PMC10778404
- DOI: 10.3390/cells13010026
Aberrations in Cell Signaling Quantified in Diabetic Murine Globes after Injury
Abstract
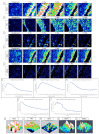
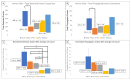
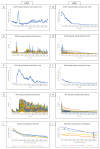
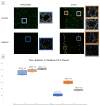
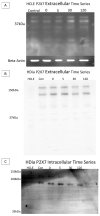
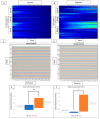
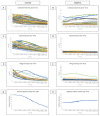
The corneal epithelium is an avascular structure that has a unique wound healing mechanism, which allows for rapid wound closure without compromising vision. This wound healing mechanism is attenuated in diabetic patients, resulting in poor clinical outcomes and recurrent non-healing erosion. We investigated changes in cellular calcium signaling activity during the wound response in murine diabetic tissue using live cell imaging from both ex vivo and in vitro models. The calcium signaling propagation in diabetic cells was significantly decreased and displayed altered patterns compared to non-diabetic controls. Diabetic cells and tissue display distinct expression of the purinergic receptor, P2X7, which mediates the wound healing response. We speculate that alterations in P2X7 expression, interactions with other proteins, and calcium signaling activity significantly impact the wound healing response. This may explain aberrations in the diabetic wound response.
Keywords: cell signaling; cornea; diabetes; live cell imaging; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Diverse calcium signaling profiles regulate migratory behavior in avascular wound healing and aberrant signal hierarchy occurs early in diabetes.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C1051-C1072. doi: 10.1152/ajpcell.00249.2024. Epub 2024 Aug 12. Am J Physiol Cell Physiol. 2024. PMID: 39129489
-
Experimental modeling of cornea wound healing in diabetes: clinical applications and beyond.BMJ Open Diabetes Res Care. 2019 Nov 27;7(1):e000779. doi: 10.1136/bmjdrc-2019-000779. eCollection 2019. BMJ Open Diabetes Res Care. 2019. PMID: 31803484 Free PMC article. Review.
-
Purinoreceptor P2X7 Regulation of Ca(2+) Mobilization and Cytoskeletal Rearrangement Is Required for Corneal Reepithelialization after Injury.Am J Pathol. 2016 Feb;186(2):285-96. doi: 10.1016/j.ajpath.2015.10.006. Epub 2015 Dec 10. Am J Pathol. 2016. PMID: 26683661 Free PMC article.
-
The roles of calcium signaling and ERK1/2 phosphorylation in a Pax6+/- mouse model of epithelial wound-healing delay.BMC Biol. 2006 Aug 16;4:27. doi: 10.1186/1741-7007-4-27. BMC Biol. 2006. PMID: 16914058 Free PMC article.
-
Purinergic Signaling in Corneal Wound Healing: A Tale of 2 Receptors.J Ocul Pharmacol Ther. 2016 Oct;32(8):498-503. doi: 10.1089/jop.2016.0009. Epub 2016 Sep 19. J Ocul Pharmacol Ther. 2016. PMID: 27643999 Free PMC article. Review.
Cited by
-
Therapeutic potential of microalgae-derived natural compounds in diabetic wound healing: A comprehensive review.Heliyon. 2025 Feb 14;11(4):e42723. doi: 10.1016/j.heliyon.2025.e42723. eCollection 2025 Feb 28. Heliyon. 2025. PMID: 40040991 Free PMC article. Review.
-
Diverse calcium signaling profiles regulate migratory behavior in avascular wound healing and aberrant signal hierarchy occurs early in diabetes.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C1051-C1072. doi: 10.1152/ajpcell.00249.2024. Epub 2024 Aug 12. Am J Physiol Cell Physiol. 2024. PMID: 39129489
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

